
(Mark One)
[X] Quarterly Report Pursuant to Section 13 or
15(d) of the Secutities Exchange Act of 1934
For the quarterly period ended September 30,
2002, or
[ ] Transition Report Pursuant to
Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from
to
Commission file number 0-21615
Massachusetts
04-2652826
(State or other Jurisdiction of
(I.R.S. Employer
Incorporation or Organization)
Identification No.)
375 West Street,
West Bridgewater, Massachusetts
02379-1040
(Address of Principal Executive Offices)
(Zip Code)
Registrant’s telephone number, including area
code
(508) 580-1900
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ]
The number of shares outstanding of the Registrant’s common stock as of November 1, 2002, was 6,786,335.

Part I — FINANCIAL INFORMATION
Item 1. Financial Statements
Consolidated Statements of Operations:
Three and Nine Months Ended September 30, 2002
Balance Sheet
Statement of Cash Flows
Notes to Financial Statements
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Item 4. Controls and Procedures
Part II — OTHER INFORMATION
Item 1. Legal Proceedings
Item 2. Changes in Securities and Use of Proceeds
Item 3. Defaults Upon Senior Securities
Item 4. Submission of Matters to a Vote of Security Holders
Item 5. Other Information
Item 6. Exhibits and Reports on Form 8-K
Signatures and Certifications
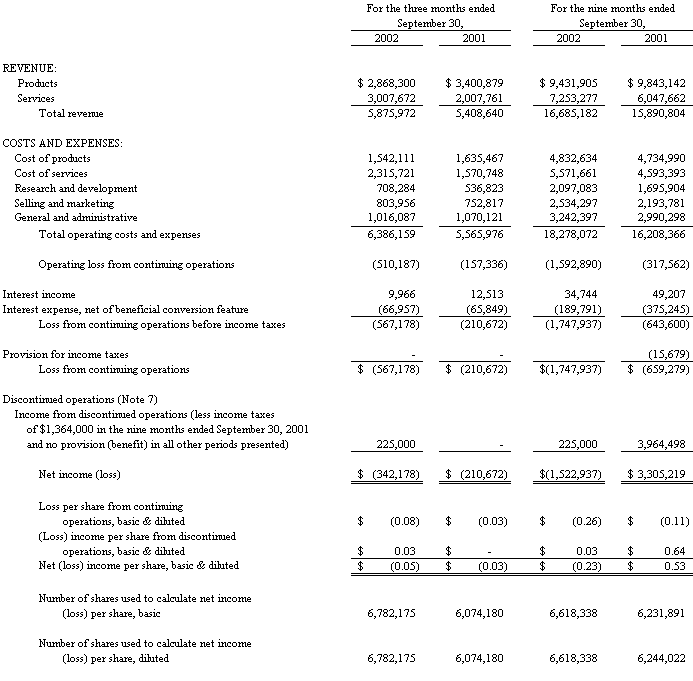
The accompanying notes are an integral part of the Consolidated Financial Statements
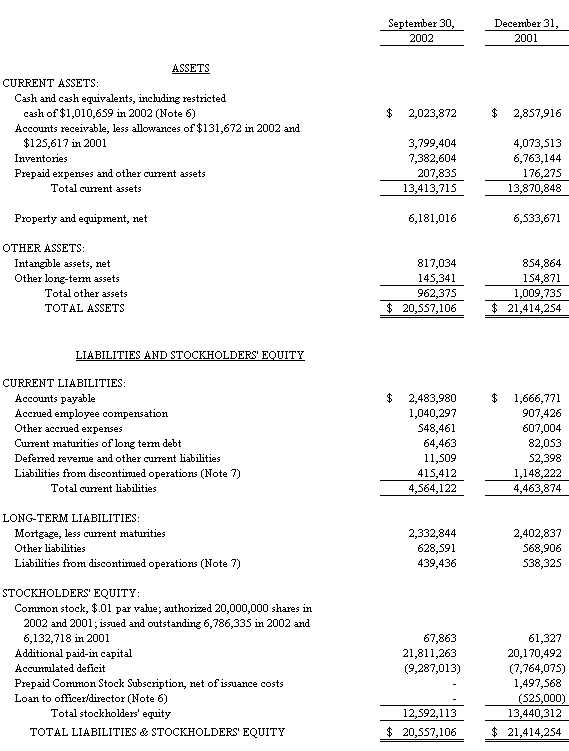
The accompanying notes are an integral part of the Consolidated Financial Statements
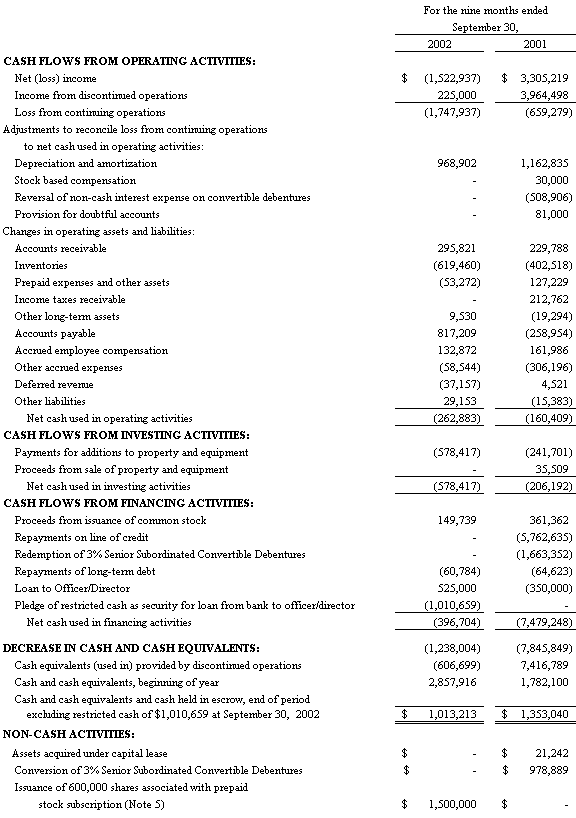
The accompanying notes are an integral part of the Consolidated Financial Statements
The accompanying unaudited consolidated financial statements have been prepared in accordance with generally accepted accounting principles for the interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by generally accepted accounting principles for complete financial statements. In the opinion of management, all adjustments (consisting of only normal recurring adjustments) considered necessary for a fair presentation have been included. Operating results for the three and nine months ended September 30, 2002 are not necessarily indicative of the results that may be expected for the year ending December 31, 2002. For further information, refer to the consolidated financial statements and footnotes thereto included in the Form 10-K filing for the fiscal year ended December 31, 2001 for Boston Biomedica, Inc. and Subsidiaries (“the Company” or “Boston Biomedica”) and the Company’s Form 10-Q filings for the three months ended March 31, 2002 and three and six months ended June 30, 2002, respectively.
Certain amounts included in the prior year’s financial statements have been reclassified to conform to the current year’s presentation.
In February 2001, the Company sold the business and certain assets and liabilities of BBI Clinical Laboratories, Inc. (“BBICL”), a wholly-owned subsidiary of the Company, to a third party in conjunction with its decision to exit the clinical laboratory business segment. The accompanying financial statements present BBICL’s remaining net liabilities and results of operations as discontinued operations.
Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets” (SFAS 142), became effective for the Company beginning January 1, 2002. SFAS 142 requires, among other things, the cessation of the amortization of goodwill. In addition, the standard includes provisions for the reclassification of certain existing recognized intangibles as goodwill, reassessment of the useful lives of existing recognized intangibles, reclassification of certain intangibles out of previously reported goodwill and the identification of reporting units for purposes of assessing potential future impairments of goodwill. SFAS 142 also required the Company to complete a transitional goodwill impairment test six months from the date of adoption. This test was performed in the second quarter and resulted in no goodwill impairment. In accordance with the adoption of this pronouncement, the Company ceased amortization of approximately $227,000 of goodwill (approximately $5,400 quarterly) attributable to its BBI Source Scientific, Inc. subsidiary. The Company’s remaining intangible assets as of January 1, 2002 relate to trademarks, license and patent costs, and accordingly, amortization of such costs over their remaining estimated useful lives is included in the accompanying financial statements for all periods presented.
Statement of Financial Accounting Standards No. 143, "Accounting for Asset Retirement Obligations" (SFAS 143), is effective January 1, 2003. SFAS 143 addresses the financial accounting and reporting for obligations and retirement costs related to the retirement of tangible long-lived assets. The adoption of SFAS 143 is not expected to have a significant impact on the Company’s financial statements.
Statement of Financial Accounting Standards No. 144, "Accounting for the Impairment or Disposal of Long-Lived
Assets" (SFAS 144), became effective January 1, 2002. SFAS 144 supersedes FASB Statement No. 121, "Accounting for
the Impairment of Long-Lived Assets and for Long-Lived Assets to Be Disposed Of," and the accounting and
reporting provisions relating to the disposal of long-lived assets. The adoption of SFAS 144 did not have a
significant impact on the Company’s financial statements.
In July 2002, the FASB issued SFAS 146, "Accounting for Costs Associated with Exit or Disposal Activities." SFAS 146 requires that a liability for a cost associated with an exit or disposal activity be recognized at its fair market value when the liability is incurred, rather than at the date of an entity's commitment to an exit plan. The provisions of SFAS 146 are effective for exit or disposal activities that are initiated after December 31, 2002. The adoption of SFAS 146 is not expected to have a material effect on the Company's financial statements.

Operating segments are components of an enterprise for which separate financial information is available that is evaluated regularly by senior management in deciding how to allocate resources and in assessing the performance of each segment. The Company is organized into segments along business lines and senior management regularly reviews financial results for all business lines, focusing primarily on revenue and operating income.
The Company had four operating segments as of September 30, 2002. The Diagnostics segment serves the worldwide in vitro diagnostics industry, including users and regulators of their test kits, with quality control products, and test kit components. The Biotech segment is a research and development center providing support for the other BBI business units, as well as contract research, molecular and cell biology services, and repository services for the government and life sciences industry. The Laboratory Instrumentation segment sells diagnostic instruments primarily to the worldwide in vitro diagnostic industry on an OEM basis, and also performs in-house instrument servicing. The PCT segment consists of research and development primarily in pressure cycling technology ("PCT"). The Company performs research in the development of PCT, with particular focus in the areas of nucleic acid extraction and pathogen inactivation. While the PCT segment’s research and development operation does not currently have any significant product or service revenue, the Company announced the availability for commercial sale of its PCT products in late September of 2002. Revenue to date in the PCT segment consists of both private and public (NIH) funding of segment research. Most of the expenditures incurred by this segment are for research and development expenses, general management expenses and patent costs. See Note 7 with respect to discontinued operations which are no longer classified as an operating segment of the Company.
The Company’s underlying accounting records are maintained on a legal entity basis for government and public reporting requirements. Inter-segment sales are recorded on a "third party best price" basis and are significant in measuring segment operating results. The following segment information has been prepared in accordance with the internal accounting policies of the Company, as described above.
Operating segment revenue was as follows:
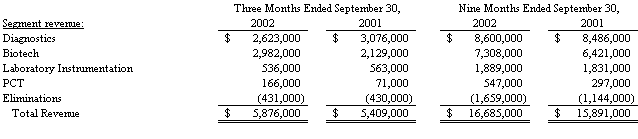
Operating segment (loss) income was as follows:

Identifiable corporate and operating segment assets are all located in the United States as follows:

Basic earnings per share is computed by dividing net income (loss) by the weighted average number of common shares outstanding. Diluted earnings per share is computed by dividing net income (loss) by the weighted average number of common shares outstanding plus additional common shares that would have been outstanding if potentially dilutive common shares had been issued. For the purposes of this calculation, stock options are considered common stock equivalents in periods in which they have a dilutive effect. Stock options that are antidilutive are excluded from the calculation. Potentially dilutive securities having a net effect of 132,855 and 215,259 common shares, for the three and nine months ended September 30, 2002 and 479,839 and 951,119 common shares, for the three and nine months ended September 30, 2001, were not included in the computation of diluted earnings per share because to do so would have been antidilutive.
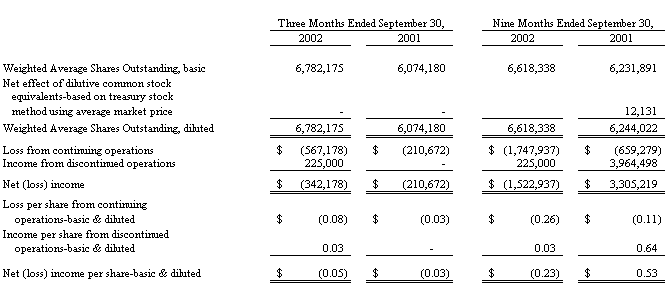
The earnings (loss) per share computation for the first nine months of 2002 reflects the issuance of 600,000 additional shares of common stock in the first quarter of 2002 to a group of investors; these shares were subscribed to and paid for in December 2001. The income (loss) per share computation for the first nine months of 2001 reflects both the issuance of 801,325 additional shares of common stock in the first quarter of 2001, as certain holders of the Debentures exercised their rights to convert $1,210,000 of such Debentures into shares of the Company’s common stock, and the issuance of 139,051 additional shares of common stock associated with the exercise of stock options and warrants, and purchases made pursuant to the employee stock purchase plan. In addition, on June 15, 2001 the Company and Paradigm Group, LLC entered into an agreement to permanently settle their disputes. Under the terms of the agreement, Paradigm Group, LLC rescinded their exercise of the common stock purchase warrants, which have since expired, and the Company retains the 500,000 shares associated with their exercise. These shares were included in the total shares outstanding as well as in the calculation of earnings (loss) per share from February 17, 2000 (the date of exercise) through June 15, 2001 (the date of the agreement). In the third quarter of 2001 the Company took the necessary measures to cancel these shares and restore these shares to authorized only status.
As of December 31, 2001, the Company had entered into a one year loan of $525,000 to its Chief Executive Officer (“CEO”), renewable at the Company’s option, and collateralized by 90,000 of his shares of Boston Biomedica common stock. Interest on the loan was payable monthly at the annual rate of 7%. The loan is shown on the balance sheet as a decrease to stockholders equity as of December 31, 2001. In January 2002, the loan was repaid in full. The loan was replaced by the Company’s pledge of a $1,000,000 interest bearing deposit at a financial institution to provide additional security for loans in the aggregate amount of $2,418,000 from the financial institution to an entity controlled by the CEO. The loans are personally guaranteed by the CEO. The Company’s pledge is secured by a junior subordinated interest in the collateral provided by the CEO to the financial institution. Such collateral includes all of his real property and common stock holdings in Boston Biomedica, Inc. The original loan and subsequent pledge of $1,000,000 were made to assist the CEO in refinancing indebtedness related to, among other things, his divorce settlement and to enable him to avoid the need to sell his common stock holdings in Boston Biomedica, Inc. on the open market to satisfy his debts. The Company’s Board of Directors and, with respect to the decision to pledge the $1,000,000 cash collateral, a special committee of the independent directors, evaluated a number of alternatives and concluded that the original loan to the CEO and the subsequent pledge were the best alternative and in the best interests of the Company’s stockholders because it would, among other things, avoid selling pressure on the Company’s common stock and relieve the financial pressures on the CEO that could otherwise divert his attention from the Company. The Company’s pledge of the $1,000,000 deposit is reflected on the Company’s balance sheet as restricted cash.
In February 2001, the Company exited the clinical laboratory testing services market and sold the business and certain assets of BBI Clinical Laboratories, Inc., a wholly-owned subsidiary of the Company, to a third party for an adjusted purchase price of $8,958,000. The Company has retained certain other assets and liabilities of BBICL, primarily property, plant and equipment, together with the facility lease subsequent to the closing date, which the Company is attempting to sublease. The Company has written down all of the retained assets not otherwise redistributed to other business units to their estimated net realizable value. In accordance with a transition services agreement, the Company operated the clinical laboratory testing business on behalf of the buyer during the period February 20, 2001 through December 2001, although most operations ceased activity by the end of June 2001; substantially all costs associated with operating the business subsequent to the closing date were borne by the purchaser.
The Company adjusted its estimated liabilities based upon third quarter developments and accordingly, the Company revised its estimate of accrued liabilities to exit the clinical laboratory testing business. The liability was reduced to $855,000 as of September 30, 2002. The major component of this remaining accrual is estimated lease exit and facility related costs ($532,000) with the remainder for health care claims, other regulatory audit adjustments, and for other miscellaneous costs associated with exiting this business segment.
The Company recorded a gain of $4,334,000 net of taxes of $969,000 in the fiscal year ended 2001,and recorded an additional after tax gain of $225,000 in the third quarter of 2002; the gain may be subject to future adjustments as the Company completes the process of exiting this business and permanently closing the facility. The remaining closing costs include an estimate to dispose of any remaining assets and retire all existing liabilities including the facility lease. The Company utilized certain prior period net operating loss carryforwards, previously reserved for by the Company, to partially offset the income tax effect of this gain. All financial data presented in the accompanying financial statements reflects discontinued operations of this segment of the business for all periods presented.
In summary, revenues from discontinued operations, net of intercompany eliminations, were $973,000, in the period
January 1, 2001 to February 20, 2001. Operating income (loss) from discontinued operations were $0 for the three
and nine months ended September 30, 2002 and $0 and ($136,000) for the three and nine months ended September 30,
2001, respectively. Income (loss) from discontinued operations was $225,000 for the three and nine months ended
September 30, 2002 and $0 and $3,964,000 for the three and nine months ended September 30, 2001 respectively.
In February 2001, the Company exited the clinical laboratory testing services market and sold the business and certain assets of BBI Clinical Laboratories, Inc., a wholly-owned subsidiary of the Company, to a third party for an adjusted purchase price of $8,958,000. Additional information relative to this transaction is contained hereunder in the caption entitled “Discontinued Operations” included in Results of Operations for the three and nine months ended September 30, 2002 and 2001.
The significant accounting policies utilized by the Company in the preparation of the accompanying financial statements are set forth in Part I, Item 7 of the Company’s Form 10-K for the year ended December 31, 2001, under the heading "Management's Discussion and Analysis of Financial Condition and Results of Operations”. There have been no changes to these policies since December 31, 2001, except as noted in the following paragraph.
Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets” (SFAS 142), became effective for the Company beginning January 1, 2002. SFAS 142 requires, among other things, the cessation of the amortization of goodwill. In addition, the standard includes provisions for the reclassification of certain existing recognized intangibles as goodwill, reassessment of the useful lives of existing recognized intangibles, reclassification of certain intangibles out of previously reported goodwill and the identification of reporting units for purposes of assessing potential future impairments of goodwill. SFAS 142 also required the Company to complete a transitional goodwill impairment test six months from the date of adoption. This test was performed in the second quarter and resulted in no goodwill impairment. In accordance with the adoption of this pronouncement, the Company ceased amortization of approximately $227,000 of goodwill (approximately $5,400 quarterly) attributable to its BBI Source Scientific, Inc. subsidiary. The Company’s remaining intangible assets as of January 1, 2002 relate to trademarks, license and patent costs, and accordingly, amortization of such costs over their remaining estimated useful lives is included in the accompanying financial statements for all periods presented.
Revenue
Total revenue from continuing operations increased 8.6%, or $467,000, to $5,876,000 in the third quarter of 2002 from $5,409,000 in the third quarter of 2001. The increase in revenue was the result of a decrease in product revenue of 15.7%, or $533,000, to $2,868,000 in the third quarter of 2002 from $3,401,000 in the third quarter of 2001, offset by a 49.9% increase in service revenue or $1,000,000, to $3,008,000 in the third quarter of 2002 from $2,008,000 in the third quarter of 2001.
Product Revenue. The decline in product revenue of the Diagnostics segment was due primarily to postponed orders as the current economic downturn in the in-vitro diagnostic industry hurt customer test kit sales and new test development.
Service Revenue. The $1,000,000 increase in service revenue was related to strong activity in two service contracts related to HIV vaccine development and Hepatitis C work at the Biotech segment; the former resulted in increased revenue for the Biotech segment as the result of increased activity from a subcontractor.
Gross Profit
Overall gross profit decreased 8.4%, or $184,000, to $2,018,000 in the third quarter of 2002 from $2,202,000 in the third quarter of 2001. Product gross profit decreased 24.9%, or $439,000, to $1,326,000 in the third quarter of 2002 from $1,765,000 in the third quarter of 2001; product gross margin declined to 46.2% in the third quarter of 2002 from 51.9% in the third quarter of 2001. Services gross profit increased $255,000 or 58.3% to $692,000 in the third quarter of 2002 from $437,000 in the third quarter of 2001, while service gross margin increased to 23.0% in the third quarter of 2002 from 21.8% in the third quarter of 2001.
Product Gross Margin
The decrease in product gross margin at the Diagnostics segment was primarily due to an unfavorable product mix shift coupled with a 15.7% decrease in product revenue.
Service Gross Margin
The service gross margin increase was due to increased service revenues at the Biotech segment associated with increased work on two vaccine contracts.
Research and Development
Research and development expenditures increased 31.8%, or $171,000, to $708,000 in the third quarter of 2002 from $537,000 in the third quarter of 2001. The increased level of expenditures was associated with ongoing PCT related projects including optimization protocols for various tissue types. In addition, there was an increase in development work on AccuChartPlus, a web based data management software for AccuRun customers.
Selling and Marketing
Selling and marketing expenses increased by 6.8%, or $51,000, to $804,000 in the third quarter of 2002 from $753,000 in the third quarter of 2001, as the Company continued to incur additional new sales, marketing and promotion costs associated with the commercial launch of the PCT Barocycler™.
General and Administrative
General and administrative costs decreased 5.0%, or $54,000, to $1,016,000 in the third quarter of 2002 from $1,070,000 in the third quarter of 2001, due to a one time credit associated with a telecommunications claim.
Operating Income (Loss) from continuing operations
Operating income (loss) from continuing operations amounted to $(510,000) in the third quarter of 2002 compared to an operating (loss) of $(157,000) in the third quarter of 2001. The Diagnostics segment’s operating income decreased to $206,000 in the third quarter of 2002 from $477,000 in the third quarter of 2001, associated with a 18.0% decrease in product revenue at the Diagnostics segment as explained above. The Biotech segment’s operating loss decreased to $(29,000) in the third quarter of 2002 from $(52,000) in the third quarter of 2001, due to higher revenues associated with work on two vaccine contracts. The operating loss of the PCT segment increased to $(490,000) in the third quarter of 2002 from $(417,000) in the same period of 2001 due to patent costs and increased research and development costs associated with the final phases of product development and advanced prototype manufacture, and a higher level of sales, marketing and promotion related expenses associated with the commercial launch of the PCT Barocycler™. The Laboratory Instrumentation segment’s operating loss increased to $(197,000) from $(165,000); this segment recorded a 4.8% decline in revenue associated with a lower level of instrument development services and a declining backlog, coupled with increased costs associated with a facility lease renewal effective in 2002.
Interest Expense
Net interest expense, primarily from the Company’s outstanding mortgage, was relatively unchanged in the third quarter of 2002 as compared to the third quarter of 2001.
Income Taxes
In the year 2000, the Company established a full valuation allowance for its deferred tax assets in accordance with Statement of Financial Accounting Standards No. 109 and in consideration of three consecutive years of losses; accordingly, the Company has not recognized an income tax benefit associated with the loss from continuing operations in the third quarter of 2002.
Loss from Continuing Operations
Loss from continuing operations amounted to $567,000 for the quarter ended September 30, 2002 as compared to a loss of $211,000 for the quarter ended September 30, 2001, as a result of the items discussed above.
Income from Discontinued Operations
The Company adjusted its estimate of remaining accrued liabilities to exit the clinical laboratory testing business based upon third quarter developments. The liability was reduced to $855,000 as of September 30, 2002. The major component of this remaining accrual is estimated lease exit and facility related costs ($532,000) with the remainder for health care claims, other regulatory audit adjustments, and for other miscellaneous costs associated with exiting this business segment. This resulted in recording an after tax gain of $225,000 in the third quarter of 2002.
Net Income (Loss)
The Company had a net (loss) of $(342,000) in the third quarter of 2002 as compared to a net (loss) of $(211,000) in the third quarter of 2001. The third quarter of 2002 included a gain of $225,000 from discontinued operations.
Revenue
Total revenue from continuing operations increased 5.0%, or $794,000, to $16,685,000 in the first nine months of 2002 from $15,891,000 in the first nine months of 2001. The increase in revenue was the result of a 19.9% increase in service revenue or $1,206,000, to $7,253,000 in the first nine months of 2002 from $6,047,000 in the first nine months, partially offset by a decline in product revenue of 4.2%, or $411,000, to $9,432,000 in the first nine months of 2002 from $9,843,000 in the first nine months of 2001.
Product Revenue. The decrease of $411,000 in product revenue was due primarily to postponed orders in the current economic downturn in the in-vitro diagnostic industry at the Diagnostic segment, coupled with decreases of product sales at the Biotech segment and lower instrument sales at the Laboratory Instrumentation segment (the latter segment experienced strong sales to existing customers in the first half of 2001).
Service Revenue. The $1,206,000 increase in service revenue was primarily related to strong activity in two service contracts related to HIV vaccine development and Hepatitis C work at the Biotech segment (the former resulting from increased revenue for the Biotech segment as the result of increased activity from a subcontractor), and increased grant revenue at the Company’s PCT segment.
Gross Profit
Overall gross profit decreased 4.3%, or $281,000, to $6,281,000 in the first nine months of 2002 from $6,562,000 in the first nine months of 2001. Product gross profit decreased 10.0%, or $509,000, to $4,599,000 in the first nine months of 2002 from $5,108,000 in the first nine months of 2001; product gross margin declined to 48.8% in the first nine months of 2002 from 51.9% in the first nine months 2001. Services gross profit increased to $1,682,000 in the first nine months of 2002 from $1,454,000 in the first nine months of 2001, while service gross margin decreased to 23.2% in the first nine months of 2002 from 24.0% in the first nine months of 2001.
Product Gross Margin
A decrease in product gross margin was associated with increased sales of higher margin catalog products in the first nine months of 2001 at the Diagnostics segment and higher raw material costs in the first nine months of 2002, a decrease in high margin product sales at the Biotech segment in the first nine months of 2002, and lower revenues from instrument sales over a relatively fixed cost structure (which includes increased costs associated with a facility lease renewal effective in 2002) at the Laboratory Instrumentation segment.
Service Gross Margin
The service gross margin decrease was due to increased service revenues associated with the PCT segment and increased activity associated with two service contracts related to HIV vaccine development and Hepatitis C work at the Biotech segment. This increase was offset by an unfavorable mix shift to lower margin government contracts combined with a lower level of billable hours associated with government reimbursable contract work and higher facility operating costs at the Biotech segment in the first nine months of 2002.
Research and Development
Research and development expenditures increased 23.6%, or $401,000, to $2,097,000 in the first nine months of 2002 from $1,696,000 in the first nine months of 2001. The increased level of expenditures was associated with ongoing PCT related projects including optimization protocols for various tissue types. In addition, there was an increase in development work on AccuChart Plus, a web based data management software for AccuRun customers.
Selling and Marketing
Selling and marketing expenses increased by 15.5%, or $340,000, to $2,534,000 in the first nine months of 2002 from $2,194,000 in the first nine months of 2001. The Company incurred significant marketing and promotion related costs in the first nine months of 2002 primarily associated with its introduction of the PCT Barocycler™ at the Pittsburgh Conference industry trade show and related ongoing sales, marketing and promotion efforts associated with the commercial launch of the PCT Barocycler™.
General and Administrative
General and administrative costs increased 8.4%, or $252,000, to $3,242,000 in the first nine months of 2002 from $2,990,000 in the first nine months of 2001, due primarily to higher than expected health care and facility costs incurred in the first nine months of 2002 partially offset by a one time credit associated with a telecommunications claim, whereas in the first nine months of 2001, the Company benefited by the reversal of an $80,000 legal expense accrual associated with the June 2001 legal settlement reached with Paradigm Group, LLC. In the second quarter of 2001, the Company increased its provision for doubtful accounts by $82,000 based on a significant deterioration in the financial condition of a customer in its Diagnostics segment.
Operating Income (Loss) from Continuing Operations
Operating (loss) from continuing operations amounted to $(1,593,000) in the first nine months of 2002 compared to an operating (loss) of $(318,000) in the first nine months of 2001. The Diagnostics segment’s operating income decreased to $893,000 in the first nine months of 2002 from $1,146,000 in the first nine months 2001 due to a decline in product revenues coupled with a lower product gross margin. The Biotech segment’s operating loss increased to $(405,000) in the first nine months of 2002 from $(143,000) in the first nine months of 2001, primarily due to both increased research and development costs and sales and marketing costs. The operating loss of the PCT segment increased to $(1,729,000) in the first nine months of 2002 from $(999,000) in the first nine months of 2001 due to increased research and development costs associated with the final phases of product development and advanced prototype manufacture and increased sales, promotion and marketing costs associated with the commercial launch in late September of 2002 of the PCT Barocycler™ .
Interest Expense
Net interest expense decreased to $155,000 in the first nine months of 2002 from $326,000 in the first nine months of 2001. The Company redeemed the remaining $2,040,000 (face value) of outstanding 3% Senior Subordinated Convertible Debentures (“Debentures”), which were originally issued in August 2000, plus accrued interest and a premium of $190,000 (which was charged to interest expense) in the first nine months of 2001. Interest expense in the first nine months of 2001 also included interest on the Company’s line of credit, which was terminated by the Company in February 2001. Both the first nine months of 2002 and 2001 included interest expense associated with the Company’s outstanding mortgage.
Income Taxes
In the year 2000, the Company established a full valuation allowance for its deferred tax assets in accordance with Statement of Financial Accounting Standards No. 109 and in consideration of three consecutive years of losses; accordingly, the Company has not recognized an income tax benefit associated with the loss from continuing operations in the first nine months of 2002 and 2001, as these tax assets have been fully reserved for. The Company made estimated state tax payments of approximately $16,000 in the first nine months of 2001.
Loss from Continuing Operations
Loss from continuing operations amounted to $1,748,000 for the nine months ended September 30, 2002 as compared to a loss of $659,000 for the nine months ended September 30, 2001 as a result of the items discussed above.
Discontinued Operations
On February 20, 2001, the Company sold the business and certain assets and liabilities of its wholly-owned subsidiary BBICL to a third party. The Company retained certain other assets and liabilities of BBICL, primarily property, plant and equipment, together with the facility lease subsequent to the closing date, which the Company is attempting to sublease. The Company wrote down all of the retained assets not otherwise redistributed to other business units to their estimated net realizable value.
The Company adjusted its estimate of remaining accrued liabilities to exit the clinical laboratory testing business based upon third quarter developments. The liability was reduced to $855,000 as of September 30, 2002. The major component of this remaining accrual is estimated lease exit and facility related costs ($532,000) with the remainder for health care claims, other regulatory audit adjustments, and for other miscellaneous costs associated with exiting this business segment. Accordingly, based upon this revised estimate of remaining liabilities, the Company recorded an after tax gain of $225,000 in the third quarter of 2002.
Revenues from discontinued operations, net of intercompany eliminations, were $973,000, in the period January 1, 2001 to February 20, 2001. Operating (losses) from discontinued operations was $0 for the three and nine months ended September 30, 2002 and were $0 and $(136,000) for the three and nine months ended September 30, 2001, respectively. The Company recorded a gain of $4,334,000, net of taxes of $969,000, in 2001. Income (loss) from discontinued operations was $225,000 for the three and nine months ended September 30, 2002 as discussed above, and $0 and $3,964,000 for the three and nine months ended September 30, 2001, respectively. The Company utilized prior period net operating loss carryforwards, previously reserved for by the Company in year 2000, to partially offset the tax effect of this gain. Additionally, the Company took a tax benefit of $564,000 related to stock option exercises that was not previously recorded as the Company was in a loss position; this tax benefit was recorded as a credit to additional paid-in capital in the first quarter of 2001.
In accordance with a transition services agreement, the Company operated the clinical laboratory business on behalf of the buyer during the period February 20, 2001 through December 2001 although most operations ceased activity by the end of June 2001. All of the revenues generated by, and substantially all costs associated with operating the business subsequent to the closing date of the transaction are the responsibility of the purchaser. A portion of the proceeds from this sale were used to redeem all outstanding Debentures and to retire the Company’s line of credit in the first quarter of 2001.
Net Income (Loss)
The Company had a net (loss) of ($1,523,000) in the first nine months of 2002 as compared to net income of $3,305,000 in the first nine months of 2001. The first nine months of 2002 included an after-tax gain of $225,000 from discontinued operations, whereas in the first nine months of 2001, the Company recorded an after-tax gain of $3,964,000 associated with discontinued operations.
At September 30th, 2002, the Company had cash and cash equivalents of $2,023,872, including restricted cash of $1,010,659, compared to cash of $2,857,916 at December 31, 2001. The company has had operating losses from continuing operations of $1,593,000 and $491,000 and has experienced negative cash flows from operations of $263,000 and $55,000 for the nine month period ended September 30, 2002 and the year ended December 2001, respectively. In addition, it is anticipated there may be additional working capital requirements in connection with PCT Barocycler™ sales and marketing activities. Management has met its recent historical cash flow needs by managing its working capital and utilizing proceeds from the February 2001 sale of one of its business segments. It plans to manage its future liquidity needs through cost reductions and additional selling initiatives. Towards this end, the Company implemented in late October of 2002 specific cost reduction measures in order to return the company to cash flow positive operations.
Net cash used in operations for the nine months ended September 30, 2002 was $263,000 as compared to $160,000 during the same period last year. The operational use of cash during the first nine months of 2002 was primarily the result of the year to date operating loss incurred in the first nine months of 2002 and the buildup of PCT raw materials inventory offset by an increase in trade accounts payable primarily associated with work on two large government contracts which occurred in the third quarter of 2002. The first nine months of 2001 included the receipt of an income tax refund offset by the operating loss incurred in the first nine months of 2001, a buildup of inventory, the reversal of non-cash interest expense and declines in both accounts payable and accrued expenses.
Net cash used in investing activities for the nine months ended September 30, 2002 was $578,000 compared to $206,000 during the same period last year. The increase of cash used for investing in the first nine months of 2002 was due to the purchase of a DNA Sequencer at the Company’s Biotech subsidiary and the construction of several preproduction PCT Barocyclers™ as demonstration units.
Cash used in financing activities for the nine months ended September 30, 2002 was $397,000 compared to cash used of $7,479,000 during the same period last year. As discussed further below, the Company pledged $1,000,000 via a deposit in an interest bearing account at a financial institution in the first nine months of 2002; this was partially offset by repayment to the Company of a loan by its Chief Executive Officer (“CEO”). In the first nine months of 2001, the Company used proceeds from the sale of certain assets of BBICL to pay off in full the remaining $5,762,635 balance on its line of credit and all remaining outstanding 3% Senior Subordinated Convertible Debentures and issued a loan to the CEO as discussed further hereunder.
As of December 31, 2001, the Company had entered into a one year loan of $525,000 to its CEO, renewable at the Company’s option, and collateralized by 90,000 of his shares of Boston Biomedica common stock; $350,000 had been loaned as of September 30, 2001. Interest on the loan was payable monthly at the annual rate of 7%. As of December 31, 2001, the loan is shown on the balance sheet as a decrease to stockholders equity. In January 2002, the loan was repaid in full. The loan was replaced by the Company’s pledge of a $1,000,000 interest bearing deposit at a financial institution to provide additional security for loans in the aggregate amount of $2,418,000 from the financial institution to an entity controlled by the CEO. The loans are personally guaranteed by the CEO. The Company’s pledge is secured by a junior subordinated interest in the collateral provided by the CEO to the financial institution. Such collateral includes all of his real property and common stock holdings in Boston Biomedica, Inc. The original loan and subsequent pledge of $1,000,000 were made to assist the CEO in refinancing indebtedness related to, among other things, his divorce settlement and to enable him to avoid the need to sell his common stock holdings in Boston Biomedica, Inc. on the open market to satisfy his debts. The Company’s Board of Directors and, with respect to the decision to pledge the $1,000,000 cash collateral, a special committee of the independent directors, evaluated a number of alternatives and concluded that the original loan to the CEO and the subsequent pledge were the best alternative and in the best interests of the Company’s stockholders because it would, among other things, avoid selling pressure on the Company’s common stock and relieve the financial pressures on the CEO that could otherwise divert his attention from the Company.
Based on current forecasts, management believes that the company has sufficient liquidity to finance operations for the next 12 months. Management's forecasts involve assumptions that could prove to be incorrect. If the company continues to incur operating losses or negative cash flows, it may need to raise additional funds. There can be no assurance that these funds will be available when required on terms acceptable to the company, if at all. If adequate funds are not available when needed, the Company may be required to further reduce its fixed costs and delay, scale back, or eliminate certain of its activities, any of which could have a material adverse long term effect on its business, financial condition or results of operations. The Company is considering various sources of additional financing, including but not limited to, strategic alliances and private placements of debt or equity securities, which could result in dilution to the Company's stockholders. On October 25, 2002, the Company retained an investment banking firm to advise the Company in the evaluation of strategic opportunities aimed at increasing shareholder value and liquidity by increasing the capital needed for growth.
As of September 30, 2002, there have been no significant changes in the Company’s contractual obligations previously disclosed as of December 31, 2001.
This Quarterly Report on Form 10-Q contains forward-looking statements concerning the Company's financial performance and business operations. The Company wishes to caution readers of this Quarterly Report on Form 10-Q that actual results might differ materially from those projected in any forward-looking statements.
Factors which might cause actual results to differ materially from those projected in the forward-looking statements contained herein include the following: due to operational, scientific or technical difficulties in the implementation of its strategies and changes in customer demand, the Company’s sales to IVD test kit manufacturers and sales of ACCURUN and other quality control products may decline; the Company may not be successful in developing Pressure Cycling Technology into commercially viable products and services, including those in the areas of sample preparation and inactivation, or such activities may take longer than currently expected; demand for commercial applications of PCT may not materialize as expected; Pressure Cycling Technology may also not be adaptable to any other commercially viable applications; certain Pressure Cycling Technology applications may not fall within the claims of the Company's eight issued U.S. patents; individuals and groups utilizing Pressure Cycling Technology may not be required to license such technology from the Company; the Company’s inability to develop the end-user market for quality control products; the Company’s inability to grow the sales of Source Scientific, Inc. to the extent anticipated; the uncertainty of the renewal and full funding of contracts with National Institutes of Health (NIH); the Company’s inability to obtain an adequate supply of the unique and rare specimens of plasma and serum necessary for certain of its products; the potential for significant reductions in purchases by any of the Company's major customers; and if expenses are higher than anticipated, or if revenues are lower than anticipated, the Company will require additional capital sooner than expected and there can be no assurance that the Company will be able to obtain additional financing or capital, or that it will be successful in eliminating or scaling back certain of its activities. Certain of these and other factors which might cause actual results to differ materially from those projected are more fully set forth under the caption "Risk Factors" in the Company's most recent Registration Statements on Form S-3 (SEC File No.’s 333-94379 and 333-46426) and in its annual report on Form 10-K for the year ended December 31, 2001 and its quarterly reports on Form 10-Q for the quarter ended March 31, 2002 and for the quarter ended June 30, 2002.
There have been no material changes in the reported market risks since December 31, 2001.
Within the 90-day period prior to the date of this report, the Company’s Chief Executive Officer and Principal Financial Officer performed an evaluation of the effectiveness of the Company’s disclosure controls and procedures (as defined in SEC Rule 13a-14), which have been designed to ensure that material information related to the Company is timely disclosed. Based upon that evaluation, they concluded that the disclosure controls and procedures were effective.
Since the last evaluation of the Company’s internal controls and procedures for financial reporting, the Company has made no significant changes in those internal controls and procedures or in other factors that could significantly affect the Company’s internal controls and procedures for financial reporting.
Not Applicable
Not Applicable
Not Applicable
Not Applicable
Not Applicable

There were no reports filed by the Company on Form 8-K in the third quarter of 2002.
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
BOSTON BIOMEDICA, INC.
(Registrant)
Date:
November 13, 2002
By: /s/ Kevin W. Quinlan
Kevin W. Quinlan,
President and Chief Operating Officer and Treasurer
(Principal Accounting and Financial Officer)
I, Richard T. Schumacher, Chairman and Chief Executive Officer, certify that:
(1) I have reviewed this quarterly report on Form 10-Q of Boston Biomedica, Inc.
(2) Based on my knowledge, this quarterly report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this quarterly report;
(3) Based on my knowledge, the financial statements, and other financial information included in this quarterly report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the period presented in this quarterly report.
(4) The registrant's other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and we have:
(a) Designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this quarterly report is being prepared.
(b) Evaluated the effectiveness of the registrant's disclosure controls and procedures as of a date within 90 days prior to the filing date of this quarterly report (the "Evaluation Date"); and
(c) Presented in this quarterly report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date;
(5) The registrant's other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant's auditors and the audit committee of registrant's board of directors (or persons performing the equivalent function):
(a) All significant deficiencies in the design or operation of internal controls which could adversely affect the registrant's ability to record, process, summarize and report financial data and have identified for the registrant's auditors any material weaknesses in internal controls; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal controls; and
(6) The registrant's other certifying officers and I have indicated in this quarterly report whether or not there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
I, Kevin W. Quinlan, President and Chief Operating Officer and Treasurer, certify that:
(1) I have reviewed this quarterly report on Form 10-Q of Boston Biomedica, Inc.
(2) Based on my knowledge, this quarterly report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this quarterly report;
(3) Based on my knowledge, the financial statements, and other financial information included in this quarterly report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the period presented in this quarterly report.
(4) The registrant's other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and we have:
(a) Designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this quarterly report is being prepared.
(b) Evaluated the effectiveness of the registrant's disclosure controls and procedures as of a date within 90 days prior to the filing date of this quarterly report (the "Evaluation Date"); and
(c) Presented in this quarterly report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date;
(5) The registrant's other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant's auditors and the audit committee of registrant's board of directors (or persons performing the equivalent function):
(a) All significant deficiencies in the design or operation of internal controls which could adversely affect the registrant's ability to record, process, summarize and report financial data and have identified for the registrant's auditors any material weaknesses in internal controls; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal controls; and
(6) The registrant's other certifying officers and I have indicated in this quarterly report whether or not there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.