
(Mark One)
[X] Quarterly Report Pursuant to Section 13 or
15(d) of the Secutities Exchange Act of 1934
For the quarterly period ended March 31,
2002, or
[ ] Transition Report Pursuant to
Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from
to
Commission file number 0-21615
Massachusetts
04-2652826
(State or other Jurisdiction of
(I.R.S. Employer
Incorporation or Organization)
Identification No.)
375 West Street,
West Bridgewater, Massachusetts
02379-1040
(Address of Principal Executive Offices)
(Zip Code)
Registrant’s telephone number, including area
code
(508) 580-1900
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ]
The number of shares outstanding of the Registrant’s common stock as of May 1, 2002, was 6,759,676.

Part I — FINANCIAL INFORMATION
Item 1. Financial Statements
Consolidated Statements of Operations:
Three Months Ended March 31, 2002
Balance Sheet
Statement of Cash Flows
Notes to Financial Statements
Item 2. Managements Discussion and Analysis of Financial Condition and Results of Operations
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Part II — OTHER INFORMATION
Item 1. Legal Proceedings
Item 2. Changes in Securities and Use of Proceeds
Item 3. Defaults Upon Senior Securities
Item 4. Submission of Matters to a Vote of Security Holders
Item 5. Other Information
Item 6. Exhibits and Reports on Form 8-K
Signature
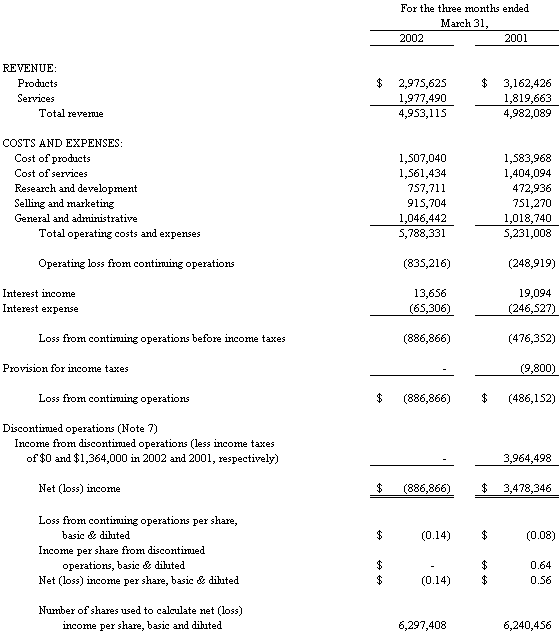
The accompanying notes are an integral part of the Consolidated Financial Statements

The accompanying notes are an integral part of the Consolidated Financial Statements
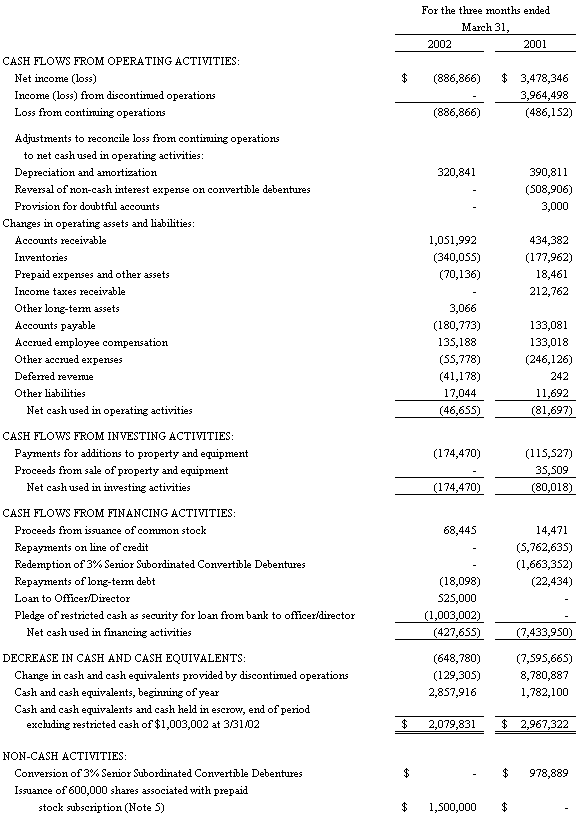
The accompanying notes are an integral part of the Consolidated Financial Statements
The accompanying unaudited consolidated financial statements have been prepared in accordance with generally accepted accounting principles for the interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by generally accepted accounting principles for complete financial statements. In the opinion of management, all adjustments (consisting of only normal recurring adjustments) considered necessary for a fair presentation have been included. Operating results for the three months ended March 31, 2002 are not necessarily indicative of the results that may be expected for the year ending December 31, 2002. For further information, refer to the consolidated financial statements and footnotes thereto included in the Form 10-K filing for the fiscal year ended December 31, 2001 for Boston Biomedica, Inc. and Subsidiaries (“the Company” or “Boston Biomedica”).
Certain amounts included in the prior year’s financial statements have been reclassified to conform to the current year’s presentation.
In February 2001, the Company sold the business and certain assets and liabilities of BBI Clinical Laboratories, Inc. (“BBICL”), a wholly-owned subsidiary of the Company, to a third party in conjunction with its decision to exit the clinical laboratory business segment. The accompanying financial statements present BBICL’s remaining net liabilities and results of operations as discontinued operations.
Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets” (SFAS 142), became effective for the Company beginning January 1, 2002. SFAS 142 requires, among other things, the cessation of the amortization of goodwill. In addition, the standard includes provisions for the reclassification of certain existing recognized intangibles as goodwill, reassessment of the useful lives of existing recognized intangibles, reclassification of certain intangibles out of previously reported goodwill and the identification of reporting units for purposes of assessing potential future impairments of goodwill. SFAS 142 also requires companies to complete a transitional goodwill impairment test six months from the date of adoption. In accordance with the adoption of this pronouncement, the Company ceased amortization of approximately $227,000 of goodwill (approximately $5,400 quarterly) attributable to its BBI Source Scientific, Inc. subsidiary. The Company’s remaining intangible assets as of January 1, 2002 relate to trademarks, license and patent costs, and accordingly, amortization of such costs over their remaining estimated useful lives is included in the accompanying financial statements for all periods presented.
Statement of Financial Accounting Standards No. 143, "Accounting for Asset Retirement Obligations" (SFAS 143), is effective January 1, 2003. SFAS 143 addresses the financial accounting and reporting for obligations and retirement costs related to the retirement of tangible long-lived assets. The adoption of SFAS 143 is not expected to have a significant impact on the Company’s financial statements.
Statement of Financial Accounting Standards No. 144, "Accounting for the Impairment or Disposal of Long-Lived Assets" (SFAS 144), became effective January 1, 2002. SFAS 144 supersedes FASB Statement No. 121, "Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to Be Disposed Of," and the accounting and reporting provisions relating to the disposal of long-lived assets. The adoption of SFAS 144 did not have a significant impact on the Company’s financial statements.

Operating segments are components of an enterprise for which separate financial information is available that is evaluated regularly by senior management in deciding how to allocate resources and in assessing the performance of each segment. The Company is organized into segments along business lines and senior management regularly reviews financial results for all business lines, focusing primarily on revenue and operating income.
The Company had four operating segments as of March 31, 2002. The Diagnostics segment serves the worldwide in vitro diagnostics industry, including users and regulators of their test kits, with quality control products, and test kit components. The Biotech segment is a research and development center providing support for the other BBI business units, as well as contract research, molecular and cell biology services, and repository services for the government and life sciences industry. The Laboratory Instrumentation segment sells diagnostic instruments primarily to the worldwide in vitro diagnostic industry on an OEM basis, and also performs in-house instrument servicing. The PCT segment consists of research and development primarily in pressure cycling technology ("PCT"). The Company performs research in the development of PCT, with particular focus in the areas of nucleic acid extraction and pathogen inactivation. While the PCT segment’s research and development operation does not currently have any significant product or service revenue, the Company expects to commercialize certain PCT products later in 2002. Revenue to date in the PCT segment consists of both private and public (NIH) funding of segment research. Most of the expenditures incurred by this segment are for research and development expenses, general management expenses and patent costs. See Note 7 with respect to discontinue operations which are no longer classified as an operating segment of the Company.
The Company’s underlying accounting records are maintained on a legal entity basis for government and public reporting requirements. Inter-segment sales are recorded on a "third party best price" basis and are significant in measuring segment operating results. The following segment information has been prepared in accordance with the internal accounting policies of the Company, as described above.
Operating segment revenue for the three months ended March 31 were as follows:

Operating segment (loss) income for the three months ended March 31 were as follows:

Identifiable corporate and operating segment assets are all located in the United States as follows:

Basic earnings per share is computed by dividing net income (loss) by the weighted average number of common shares outstanding. Diluted earnings per share is computed by dividing net income (loss) by the weighted average number of common shares outstanding plus additional common shares that would have been outstanding if dilutive potential common shares had been issued. For the purposes of this calculation, stock options are considered common stock equivalents in periods in which they have a dilutive effect. Stock options that are antidilutive are excluded from the calculation. Potentially dilutive securities having a net effect of 151,230 and 1,124,040 common shares, for the three months ended March 31, 2002 and 2001, respectively, were not included in the computation of diluted earnings per share because to do so would have been antidilutive.
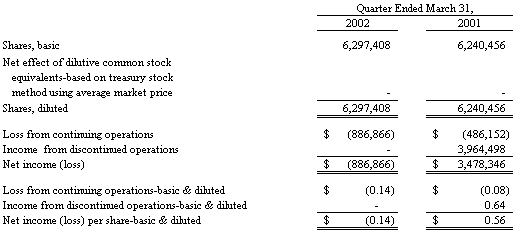
In December 2001, an additional 600,000 shares of common stock were subscribed to and paid for by a group of investors for $1,500,000. These shares were issued in the first quarter of 2002.
As of December 31, 2001, the Company had entered into a one year loan of $525,000 to its Chief Executive Officer (“CEO”), renewable at the Company’s option, and collateralized by 90,000 of his shares of Boston Biomedica common stock. Interest on the loan was payable monthly at the annual rate of 7%. The loan is shown on the balance sheet as a decrease to stockholders equity as of December 31, 2001. In January 2002, the loan was repaid in full. The loan was replaced by the Company’s pledge of a $1,000,000 interest bearing deposit at a financial institution to provide additional security for loans in the aggregate amount of $2,418,000 from the financial institution to an entity controlled by the CEO. The loans are personally guaranteed by the CEO. The Company’s pledge is secured by a junior interest in the collateral provided by the CEO to the financial institution. Such collateral includes all of his real property and common stock holdings in Boston Biomedica, Inc. The original loan and subsequent pledge of $1,000,000 were made to assist the CEO in refinancing indebtedness related to, among other things, his divorce settlement and to enable him to avoid the need to sell his common stock holdings in Boston Biomedica, Inc. on the open market to satisfy his debts. The Company’s Board of Directors and, with respect to the decision to pledge the $1,000,000 cash collateral, a special committee of the independent directors, evaluated a number of alternatives and concluded that the original loan to the CEO and the subsequent pledge were the best alternative and in the best interests of the Company’s stockholders because it would, among other things, avoid selling pressure on the Company’s common stock and relieve the financial pressures on the CEO that could otherwise divert his attention from the Company. The Company’s pledge of the $1,000,000 deposit is reflected on the Company’s balance sheet as restricted cash.
In December 2000, the Company made a decision to exit the clinical laboratory testing services segment and in February 2001, BBI Clinical Laboratories, Inc., a wholly-owned subsidiary of the Company, sold the business and certain assets and liabilities of its clinical laboratory business to a third party for an adjusted purchase price of $8,958,000. The Company has retained certain other assets and liabilities of BBICL, primarily property, plant and equipment, together with the facility lease subsequent to the closing date, which the Company is attempting to sublease. The Company has written down all of the retained assets not otherwise redistributed to other business units to their estimated net realizable value. In accordance with a transition services agreement, the Company operated the clinical laboratory testing business on behalf of the buyer during the period February 20, 2001 through December 2001, although most operations ceased activity by the end of June 2001; substantially all costs associated with operating the business subsequent to the closing date were borne by the purchaser.
The Company has recorded its estimate of remaining short and long term accrued liabilities to exit the clinical laboratory testing business, totaling approximately $1,557,000 as of March 31, 2002. The major components of this accrual are estimated income taxes ($312,000), estimated lease exit and facility related costs ($692,000) and potential health care claims and other related potential audit adjustments ($318,000), with the remainder for other miscellaneous costs associated with exiting this business segment.
The Company recorded an after-tax gain of $4,334,000 in the fiscal year ended 2001, which may be subject to future adjustments as the Company completes the process of exiting this business and permanently closing the facility. The remaining closing costs include an estimate to dispose of any remaining assets and retire all existing liabilities including the facility lease. The Company will utilize certain prior period net operating loss carryforwards, previously reserved for by the Company, to partially offset the income tax effect of this gain. All financial data presented in the accompanying financial statements reflects discontinued operations of this segment of the business for all periods presented.
In summary, revenues from discontinued operations, net of intercompany eliminations, were $973,000, in the period January 1, 2001 to February 20, 2001. Operating income (loss) from discontinued operations for the three months ended March 31, 2002 and March 31, 2001 was $0 and ($136,000), respectively. The Company recorded a gain of $4,100,000, net of taxes of $1,364,000, in the first quarter of 2001, such gain being subject to subsequent post closing adjustments. Income (loss) from discontinued operations was $0 and $3,964,000 for the three months ended March 31, 2002 and 2001, respectively.
In December 2000, the Company made a decision to exit the clinical laboratory testing services segment and in February 2001, BBI Clinical Laboratories, Inc. (“BBICL”), a wholly-owned subsidiary of the Company, sold the business and certain assets and liabilities to a third party for an adjusted purchase price of $8,958,000. Additional information relative to this transaction is contained hereunder in the caption entitled “Discontinued Operations.”
Revenue
Total revenue from continuing operations decreased 0.1%, or $29,000, to $4,953,000 in the first quarter of 2002 from $4,982,000 in the first quarter of 2001. The decrease in revenue was the result of a decrease in product revenue of 5.9%, or $187,000, to $2,975,000 in 2002 from $3,162,000 in 2001. This was partially offset by an increase in service revenue of 8.6%, or $158,000, to $1,977,000 in 2002 from $1,820,000 in 2001.
Product Revenue. The decrease of $187,000 in product revenue was due primarily to a decrease of approximately $81,000 at the Diagnostic segment and $74,000 at the Laboratory Instrumentation segment. These decreases were primarily a result of delays from several customers at the Diagnostics segment in getting final customer approval for shipment, and the impact of the Company’s increased focus on commercializing pressure cycling technology (“PCT”), which diverted resources from other projects.
Service Revenue. The $158,000 increase in service revenue was primarily related to growth in the repository operations at the Biotech segment.
Gross Profit
Overall gross profit decreased 5.5%, or $109,000, to $1,885,000 in the first quarter of 2002 from $1,994,000 for the same period last year. Product gross profit decreased 6.9%, or $109,000, to $1,469,000 in 2002 from $1,578,000 for 2001; product gross margin was relatively unchanged at 49.4% in 2002 from 49.9% in 2001. Services gross profit was flat at $416,000 in the first quarter of both 2002 and 2001 while service gross margin decreased slightly to 21.0% in 2002 from 22.8% in 2001.
Product Gross Margin
A slight increase in product gross margin at the Diagnostics segment, which was primarily due to the mix of diagnostic component products, was offset by a slight decrease at the Laboratory Instrumentation segment. The latter decrease was associated with lower revenues over a relatively fixed cost structure and related facility costs.
Service Gross Margin
The service gross margin declined slightly due to an unfavorable mix shift as the revenue increase was in lower margin government contracts.
Research and Development
Research and development expenditures increased 60.3%, or $285,000, to $758,000 in 2002 from $473,000 in 2001. The increased level of research and development expenditures in the first quarter of 2002 was primarily associated with building several PCT Barocyclers™ for use at beta sites.
Selling and Marketing
Selling and marketing expenses increased by 22.0%, or $165,000, to $916,000 in 2002 from $751,000 in 2001. The Company incurred significant marketing and promotion related costs in the first quarter of 2002 associated with its introduction of the PCT Barocycler™ at the Pittsburgh conference industry trade show.
General and Administrative
General and administrative costs increased 2.6%, or $27,000, to $1,046,000 in 2002 from $1,019,000 in 2001, due to an increase in professional fees.
Operating Loss
Operating loss from continuing operations increased to $(835,000) in 2002 versus an $(249,000) loss in 2001. The Diagnostics segment’s operating income decreased to $138,000 in 2002 from $203,000 in 2001, associated with a decline in product revenue. The Biotech segment’s operating loss increased to $(133,000) in 2002 from $(51,000) in 2001, due to increased research and development costs. The operating loss of the PCT segment increased to $(791,000) in 2002 from $(279,000) in 2001 due to approximately $113,000 in costs related to marketing and launching the Barocycler™ and related PULSE™ tubes as well as increased research and development costs associated with the final phases of product development and advanced prototype manufacture.
Interest Expense
Net interest expense decreased to $52,000 in 2002 from $227,000 in 2001. In the first quarter of 2001, the Company redeemed the remaining $2,040,000 (face value) of outstanding 3% Senior Subordinated Convertible Debentures (“Debentures”), which were originally issued in August 2000, plus accrued interest and a premium of $190,000 (which was charged to interest expense). Interest expense in the first quarter of 2001 also included interest on the Company’s line of credit, which was terminated by the Company in February 2001. Both the first quarter of 2002 and 2001 included interest expense on the Company’s outstanding mortgage.
Income Taxes
In the year 2000, the Company established a full valuation allowance for its deferred tax assets in accordance with Statement of Financial Accounting Standards No. 109 and in consideration of three consecutive years of losses; accordingly, the Company has not recognized an income tax benefit associated with the loss from continuing operations in the first quarters of 2002 and 2001, as these tax assets have been fully reserved for. The Company made estimated state tax payments of $9,800 in the first quarter of 2001.
Loss from Continuing Operations
Loss from continuing operations increased to $(887,000) for the quarter ended March 31, 2002 from $(486,000) for the same period last year, as a result of the items discussed above.
Discontinued Operations
On February 20, 2001, the Company sold the business and certain assets and liabilities of its wholly-owned subsidiary BBICL to a third party. The Company retained certain other assets and liabilities of BBICL, primarily property, plant and equipment, together with the facility lease subsequent to the closing date, which the Company is attempting to sublease. The Company wrote down all of the retained assets not otherwise redistributed to other business units to their estimated net realizable value.
The Company has recorded its estimate of remaining short and long term accrued liabilities to exit the clinical laboratory testing business, totaling approximately $1,557,000 as of March 31, 2002. The major components of this accrual are estimated income taxes ($312,000), estimated lease exit and facility related costs ($692,000) and potential health care claims and other related potential audit adjustments ($318,000), with the remainder for other miscellaneous costs associated with exiting this business segment.
Revenues from discontinued operations, net of intercompany eliminations, were $973,000, in the period January 1, 2001 to February 20, 2001. Operating income (loss) from discontinued operations for the three months ended March 31, 2002 and March 31, 2001 was $0 and $(136,000), respectively. The Company recorded a gain of $4,100,000, net of taxes of $1,364,000, in the first quarter of 2001, such gain being subject to subsequent post closing adjustments. Income (loss) from discontinued operations was $0 and $3,964,000 for the three months ended March 31, 2002 and 2001, respectively. The Company will utilize prior period net operating loss carryforwards, previously reserved for by the Company in year 2000, to partially offset the tax effect of this gain. Additionally, the Company took a tax benefit of $564,000 related to stock option exercises that was not previously recorded as the Company was in a loss position; this tax benefit was recorded as a credit to additional paid-in capital in the first quarter of 2001.
In accordance with a transition services agreement, the Company operated the clinical laboratory business on behalf of the buyer during the period February 20, 2001 through December 2001 although most operations ceased activity by the end of June 2001. All of the revenues generated by, and substantially all costs associated with operating the business subsequent to the closing date of the transaction are the responsibility of the purchaser. A portion of the proceeds from this sale were used to redeem all outstanding Debentures and to retire the Company’s line of credit in the first quarter of 2001.
Summary
The Company had a net (loss) of ($887,000) in 2002 as compared to net income of $3,478,000 in 2001. In the first quarter of 2001, the Company recorded an after-tax gain of $3,964,000 associated with discontinued operations. The earnings (loss) per share computation for the first quarter of 2002 reflects the issuance of 600,000 additional shares of common stock in the first quarter of 2002 to a group of investors; such shares were subscribed to and paid for in December 2001. The earnings (loss) per share computation in the first quarter of 2001 reflects the issuance of 801,325 additional shares of common stock, as certain holders of the Debentures exercised their rights to convert $1,210,000 of such Debentures into shares of the Company’s common stock.
The Company's working capital position amounted to $9,376,000 (including restricted cash of $1,003,000) as of March 31, 2002 from $9,407,000 as of December 31, 2001.
Net cash used in operations for the three months ended March 31, 2002 was $47,000 as compared to $82,000 during the same period last year. The operational use of cash during the first quarter of this year was primarily the result of the quarterly loss and the buildup of raw materials inventory partially offset by favorable cash collections of receivables, whereas in the first quarter of 2001, the operating loss coupled with the reversal of non-cash interest expense was partially offset by increased collections on accounts receivable, receipt of an income tax refund and a increase in accounts payable and accrued expenses.
Net cash used in investing activities was $174,000 in the first quarter of 2002 versus $80,000 in the comparable prior year period. The increase of cash used for investing in the first quarter of 2002 was due to the purchase of a DNA Sequencer at the Company’s Biotech subsidiary.
Cash used in financing activities was $428,000 in the first quarter of 2002 versus cash used of $7,434,000 for the prior year period. In the first quarter of 2002 the Company pledged $1,000,000 partially offset by repayment to the Company of a loan to the CEO also discussed further below. In the first quarter of 2001, the Company used proceeds from the sale of certain assets of BBICL to pay off in full the remaining $5,762,635 balance on its line of credit and all remaining outstanding 3% Senior Subordinated Convertible Debentures.
As of December 31, 2001, the Company had entered into a one year loan of $525,000 to its Chief Executive Officer (“CEO”), renewable at the Company’s option, and collateralized by 90,000 of his shares of Boston Biomedica common stock. Interest on the loan was payable monthly at the annual rate of 7%. As of December 31, 2001, the loan is shown on the balance sheet as a decrease to stockholders equity. In January 2002, the loan was repaid in full. The loan was replaced by the Company’s pledge of a $1,000,000 interest bearing deposit at a financial institution to provide additional security for loans in the aggregate amount of $2,418,000 from the financial institution to an entity controlled by the CEO. The loans are personally guaranteed by the CEO. The Company’s pledge is secured by a junior interest in the collateral provided by the CEO to the financial institution. Such collateral includes all of his real property and common stock holdings in Boston Biomedica, Inc. The original loan and subsequent pledge of $1,000,000 were made to assist the CEO in refinancing indebtedness related to, among other things, his divorce settlement and to enable him to avoid the need to sell his common stock holdings in Boston Biomedica, Inc. on the open market to satisfy his debts. The Company’s Board of Directors and, with respect to the decision to pledge the $1,000,000 cash collateral, a special committee of the independent directors, evaluated a number of alternatives and concluded that the original loan to the CEO and the subsequent pledge were the best alternative and in the best interests of the Company’s stockholders because it would, among other things, avoid selling pressure on the Company’s common stock and relieve the financial pressures on the CEO that could otherwise divert his attention from the Company.
As of March 31, 2002, the Company believes its cash and working capital resources (exclusive of the $1,000,000 pledge and loan guarantee discussed above), coupled with internally generated funds from operations, will be sufficient to fund operations and anticipated capital expenditures for the next year. The Company continually evaluates financing options, as well as other strategic alternatives, in order to maximize shareholder value.
As of March 31, 2002, there have been no significant changes in the Company’s contractual obligations previously disclosed as of December 31, 2001.
This Quarterly Report on Form 10-Q contains forward-looking statements concerning the Company's financial performance and business operations. The Company wishes to caution readers of this Quarterly Report on Form 10-Q that actual results might differ materially from those projected in any forward-looking statements.
Factors which might cause actual results to differ materially from those projected in the forward-looking statements contained herein include the following: due to operational, scientific or technical difficulties in the implementation of its strategies and changes in customer demand, the Company’s sales to IVD test kit manufacturers and sales of ACCURUN and other quality control products may not continue to be as strong as in 2001; the Company may not be successful in developing Pressure Cycling Technology into commercially viable products and services, including those in the areas of sample preparation and inactivation, or such activities may take longer than currently expected; Pressure Cycling Technology may also not be adaptable to any other commercially viable applications; certain Pressure Cycling Technology applications may not fall within the claims of the Company's eight issued U.S. patents; individuals and groups utilizing Pressure Cycling Technology may not be required to license such technology from the Company; the Company’s inability to develop the end-user market for quality control products; the Company’s inability to grow the sales of Source Scientific, Inc. to the extent anticipated; the uncertainty of the renewal and full funding of contracts with National Institutes of Health (NIH); the Company’s inability to obtain an adequate supply of the unique and rare specimens of plasma and serum necessary for certain of its products; the potential for significant reductions in purchases by any of the Company's major customers; and if expenses are higher than anticipated, or if revenues are lower than anticipated, the Company will require additional capital sooner than expected and there can be no assurance that the Company will be able to obtain additional capital on acceptable terms. Certain of these and other factors which might cause actual results to differ materially from those projected are more fully set forth under the caption "Risk Factors" in the Company's most recent Registration Statements on Form S-3 (SEC File No.’s 333-94379 and 333-46426).
There have been no material changes in the reported market risks since December 31, 2001.
Not Applicable
In December 2001, the Company sold 600,000 shares of common stock of the Company for an aggregate purchase price of $1,500,000 in a private placement to five accredited investors. The shares were issued in the first quarter of fiscal 2002 and therefore were not included in the total shares outstanding as well as in the calculation of earnings (loss) per share for the year ended December 31, 2001. The issuance of the shares was effected without registration under the Securities Act of 1933, as amended, in reliance upon the exemption from registration contained in Rule 506 of Regulation D promulgated under the Securities Act.
In accordance with the terms of the Company’s mortgage with a bank, payment of dividends on common stock is not permitted.
Not Applicable
Not Applicable
Not Applicable

There were not reports filed by the Company on Form 8-K in the first quarter of 2002
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
BOSTON BIOMEDICA, INC.
(Registrant)
Date:
May 14, 2002
By: /s/ Kevin W. Quinlan
Kevin W. Quinlan,
President and Chief Operating Officer
(Principal Accounting and Financial Officer)