
(Mark One)
[X] Quarterly Report Pursuant to Section 13 or
15(d) of the Secutities Exchange Act of 1934
For the quarterly period ended June 30, 2001, or
[ ] Transition Report Pursuant to
Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from
to
Commission file number 0-21615
Massachusetts
04-2652826
(State or other Jurisdiction of
(I.R.S. Employer
Incorporation or Organization)
Identification No.)
375 West Street,
West Bridgewater, Massachusetts
02379-1040
(Address of Principal Executive Offices)
(Zip Code)
Registrant’s telephone number, including area
code
(508) 580-1900
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ]
The number of shares outstanding of the Registrant’s only class of common stock as of August 3, 2001, was 6,058,502.

Part I — FINANCIAL INFORMATION
Item 1. Financial Statements
Consolidated Statements of Operations:
Three and Six Months Ended June 30, 2001
Balance Sheet
Statement of Cash Flows
Notes to Financial Statements
Item 2. Managements Discussion and Analysis of Financial Condition and Results of Operations
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Part II — OTHER INFORMATION
Item 1. Legal Proceedings
Item 2. Changes in Securities and Use of Proceeds
Item 3. Defaults Upon Senior Securities
Item 4. Submission of Matters to a Vote of Security Holders
Item 5. Other Information
Item 6. Exhibits and Reports on Form 8-K
Signature
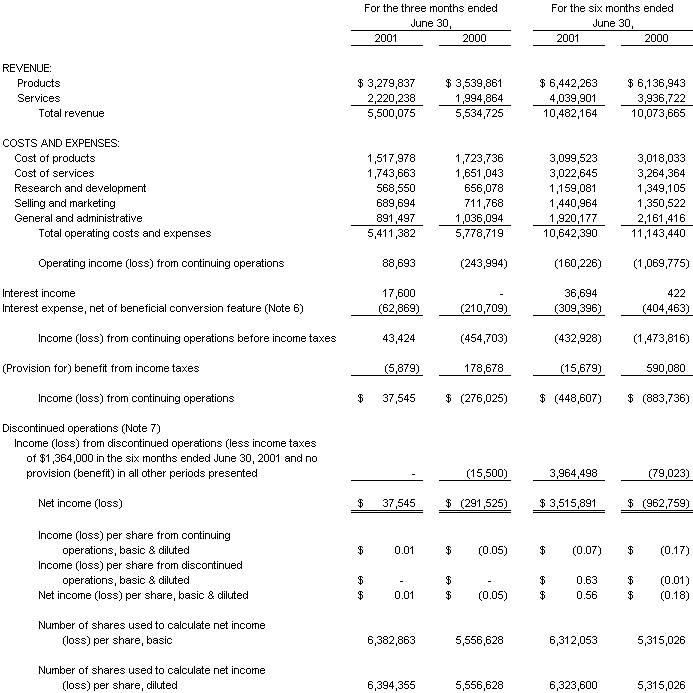
The accompanying notes are an integral part of the Consolidated Financial Statements
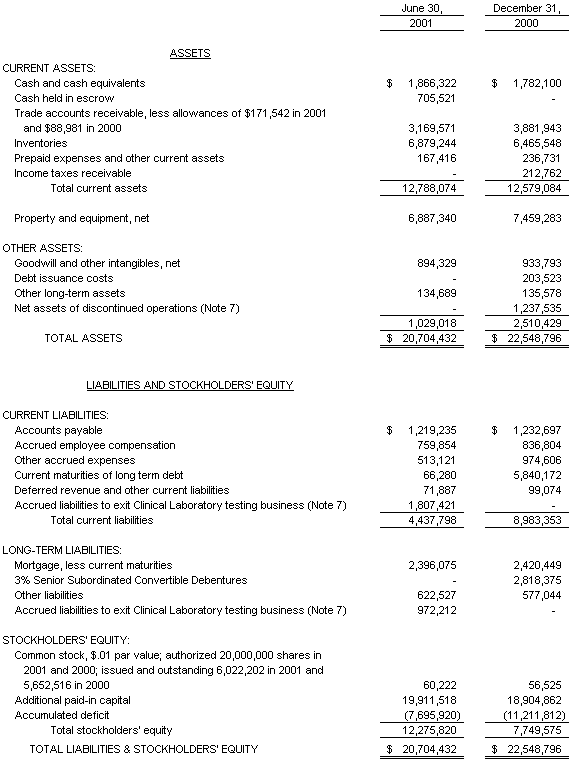
The accompanying notes are an integral part of the Consolidated Financial Statements
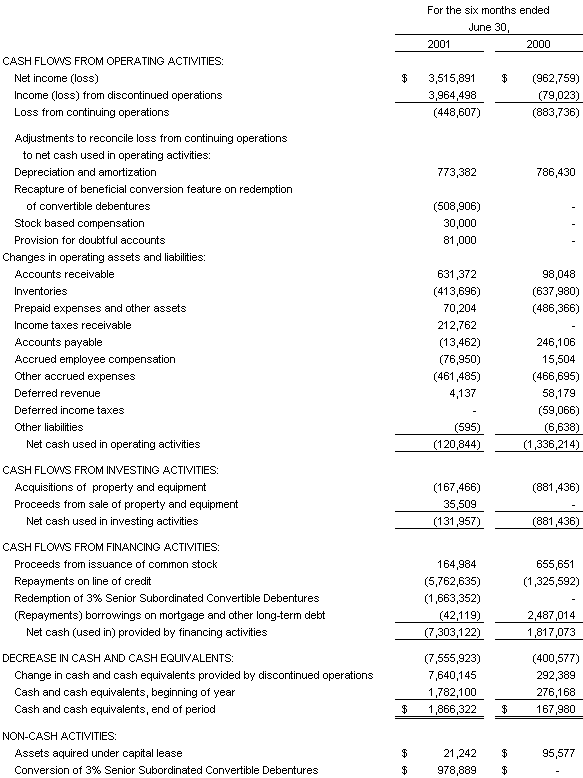
The accompanying notes are an integral part of the Consolidated Financial Statements
The accompanying unaudited consolidated financial statements have been prepared in accordance with generally accepted accounting principles for the interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by generally accepted accounting principles for complete financial statements. In the opinion of management, all adjustments (consisting of only normal recurring adjustments) considered necessary for a fair presentation have been included. Operating results for the three months and six months ended June 30, 2001 are not necessarily indicative of the results that may be expected for the year ending December 31, 2001. For further information, refer to the consolidated financial statements and footnotes thereto included in the Form 10-K/A filing for the fiscal year ended December 31, 2000 for Boston Biomedica, Inc. and Subsidiaries (“the Company” or “Boston Biomedica”) and the Company’s Form 10-Q filing for the three months ended March 31, 2001.
In February 2001, the Company sold the business and certain assets of BBI Clinical Laboratories, Inc. (“BBICL”), a wholly-owned subsidiary of the Company, to a third party in conjunction with its decision to exit the clinical laboratory testing business. Accordingly, the accompanying financial statements have been reclassified to present BBICL’s net assets and results of operations as discontinued operations.
Certain amounts included in the prior year's financial statements have been reclassified to conform to the current year’s presentation.
Statement of Financial Accounting Standards No. 133, “Accounting for Derivative Instruments and Hedging Activities” (SFAS 133), as amended, is effective for the first quarter of fiscal years beginning after June 15, 2000. The new standard requires companies to record derivatives on the balance sheet as assets or liabilities, measured at fair value. Gains or losses resulting from changes in the values of those derivatives are accounted for depending on the use of the derivatives and whether they qualify for hedge accounting. The key criterion for hedge accounting is that the hedging relationship must be highly effective in achieving offsetting changes in fair value or cash flows. The Company does not currently engage in derivative trading or hedging activities so the adoption of SFAS 133 did not have a material effect on its financial statements.
Statement of Financial Accounting Standards No. 141, “Business Combinations” (SFAS 141), is effective for all business combinations initiated after June 30, 2001. The new standard requires companies to record business combinations using the purchase method of accounting. The Company does not expect the adoption of SFAS 141 to have a material effect on its financial statements.
Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets” (SFAS 142), is effective for the Company beginning January 1, 2002. SFAS 142 requires, among other things, the cessation of the amortization of goodwill. In addition, the standard includes provisions for the reclassification of certain existing recognized intangibles as goodwill, reassessment of the useful lives of existing recognized intangibles, reclassification of certain intangibles out of previously reported goodwill and the identification of reporting units for purposes of assessing potential future impairments of goodwill. SFAS 142 also requires companies to complete a transitional goodwill impairment test six months from the date of adoption. We are currently assessing the impact of this new statement on our consolidated financial position and results of operations but do not believe that its adoption will have a material effect on the Company’s financial statements.

Operating segments are components of an enterprise for which separate financial information is available that is evaluated regularly by senior management in deciding how to allocate resources and in assessing the performance of each segment. The Company is organized into segments along legal entity lines and senior management regularly reviews financial results for all entities, focusing primarily on revenue and operating income.
The Company had four operating segments as of June 30, 2001: Diagnostics; Biotech; Laboratory Instrumentation and Pressure Cycling Technology (“PCT”). The Company has split its “Other” segment into two components; PCT and Panacos. For most of fiscal year 2000 the Company also had a drug discovery segment which was Panacos Pharmaceuticals. In November 2000, Panacos sold a majority of its equity to third party investors and as a result, the Company no longer consolidates the results of Panacos in its financial reporting. The Diagnostics segment serves the worldwide in vitro diagnostics industry, including end users and regulators of their test kits, with quality control products and test kit components. The Biotech segment provides research and development support for the other BBI segments as well as contract research and repository services for agencies of the United States Government, industry and other third parties. The Laboratory Instrumentation segment sells diagnostic instruments and medical devices primarily to the worldwide in vitro diagnostic industry on an OEM basis, and also performs in-house instrument servicing. The PCT segment conducts research and development using the Company’s patented Pressure Cycling Technology, with particular focus in the areas of nucleic acid purification and pathogen inactivation. This segment does not currently have any significant product or service revenue. The revenue to date consists of both private and public funding of specific research projects. Most of the expenditures by this segment are for R&D expenses and general management expenses, including patent costs.
The Company’s underlying accounting records are maintained on a legal entity basis for government and public reporting requirements, as well as for segment performance and internal management reporting. Inter-segment sales are recorded on a "third party best price" basis and are significant in measuring segment operating results. The following segment information has been prepared in accordance with the internal accounting policies of the Company, as described above. Prior year data has been restated to conform to the current year presentation format.
Operating segment revenue was as follows:
In the first quarter of 2001, the Company adjusted its absorption of corporate overhead based upon a revised corporate structure effective in year 2001. The present corporate structure reflects the Company’s implementation, in the latter part of year 2000, of a cost reduction plan at the Laboratory Instrumentation segment, the Company’s spin-off of Panacos as an independent company, and the Company’s decision to exit the Clinical Laboratory testing business. The latter item is reflected as discontinued operations in the accompanying financial statements. In accordance with generally accepted accounting principles, the Company ceased allocating corporate overhead to the Clinical Laboratory testing business for all periods presented. This adjustment results in the Diagnostics unit absorbing a large portion of corporate overhead which in prior years would have been allocated to Panacos and the Clinical Laboratory testing business.
Segment operating income (loss) was as follows:

Basic earnings (loss) per share is computed by dividing net income (loss) by the weighted average number of common shares outstanding. Diluted earnings (loss) per share is computed by dividing net income (loss) by the weighted average number of common shares outstanding plus additional common shares that would have been outstanding if dilutive potential common shares had been issued. For the purposes of this calculation, stock options are considered common stock equivalents in periods in which they have a dilutive effect. Stock options that are antidilutive are excluded from the calculation. Potentially dilutive stock options having a net effect of 1,121,695 and 371,071 common shares, for the three months ended June 30, 2001 and 2000, respectively, and 1,033,755 and 585,493 common shares, for the six months ended June 30, 2001 and 2000, respectively, were not included in the computation of diluted earnings per share because to do so would have been antidilutive. Additionally, 336,345 and 343,181 common stock purchase warrants, outstanding at June 30, 2001 and 2000, respectively were not included in the computation of diluted earnings per share because to do so would have been antidilutive. Included in total shares outstanding for the period February 17, 2000 to June 15, 2001 are 500,000 shares associated with the exercise of certain warrants by Paradigm Group, LLC, as discussed further on Note 8 hereunder.
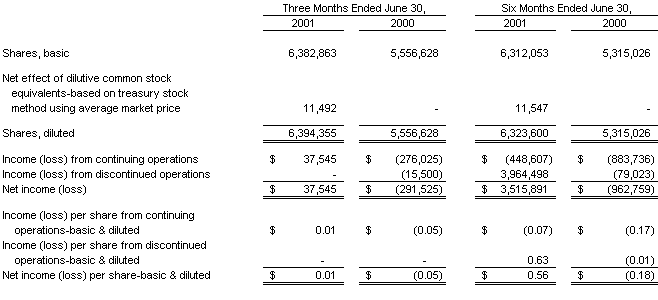
In February 2001, the Company utilized a portion of the proceeds from the sale of its Clinical Laboratory business segment to pay off in full the outstanding balance of $5,762,635 (plus accrued interest) on its line of credit, at which time the bank released all liens associated with this line of credit and terminated the line of credit.
In the first quarter of 2001, certain holders of the Company’s 3% Senior Subordinated Convertible Debentures (the “Debentures”) exercised their rights to convert $1,210,000 of such Debentures into shares of the Company’s common stock. These conversions resulted in the issuance of 801,325 shares of common stock. The pro-rata portion of the unamortized original issue discount, debt issuance and warrant related costs amounting to approximately $231,000 was charged against additional paid-in capital. In addition, the Company redeemed the remaining $2,040,000 (face value) of Debentures plus accrued interest and a premium of $190,000 (which was charged to interest expense). The pro-rata portion of unamortized original issue discount, debt issuance and warrant related costs associated with the redeemed Debentures amounting to approximately $377,000 has been included in the loss on extinguishment of the Debentures which is included in interest expense. Partially offsetting this loss is the Company’s reversal of approximately $528,000 of interest expense previously recorded in 2000 associated with the amortization of the Debentures’ beneficial conversion feature. Accordingly, the Company has recorded a loss of approximately $(39,000) relative to the early extinguishment of the Debentures.
As a result of the conversions and redemptions noted above, none of the Debentures remain outstanding subsequent to February 27, 2001.
On February 20, 2001, BBI Clinical Laboratories, Inc. (“BBICL”), a wholly-owned subsidiary of the Company, sold certain assets and liabilities of its clinical laboratory business to a third party for an original purchase price of $9,500,000, of which $900,000 was placed in escrow subject to certain post closing adjustments. The escrow amount has been adjusted by $206,000 to reflect a post closing adjustment in favor of the purchaser and for $12,000 interest earned. The Company has retained certain other assets and liabilities of BBICL, primarily property plant and equipment together with a facility lease subsequent to the closing date, which the Company intends to liquidate throughout the remainder of year 2001 as part of its decision to exit this segment of the business. The Company has written all of the retained assets down to their estimated net realizable value. In accordance with a transition services agreement, the Company is required to operate the business in a normal fashion for a minimum of six months subsequent to the sale but in no event beyond December 31, 2001; all of the revenues generated by, and substantially all costs associated with operating the BBICL business subsequent to the closing date of this transaction will belong to the purchaser.
The Company has recorded a gain of $4,100,000 net of taxes of $1,364,000 in the first quarter of 2001, subject to post closing adjustments. The Company expects to utilize approximately $5,200,000 of prior period net operating loss carryforwards, previously reserved for by the Company in year 2000, to partially offset the tax effect of this gain. Additionally, the Company has taken a tax benefit of $364,000 related to stock option exercises that was not previously recorded as the Company was in a loss position. This tax benefit was recorded as a credit to additional paid-in capital in the first half of 2001.
As of June 30, 2001, the Company’s most recent estimate of its remaining short and long term accrued liabilities to exit the Clinical Laboratory testing business, is approximately $2,780,000. The major components of this accrual are estimated income taxes ($817,000), severance and other employee related costs ($669,000), estimated lease exit costs ($773,000) and potential health care claim adjustments ($218,000); with the remainder for professional fees, potential additional post-closing adjustments, and other miscellaneous costs associated with exiting this business segment.
Revenues from discontinued operations net of intercompany eliminations were $973,000 for the period from January 1, 2001 to February 20, 2001 and $2,219,000 and $4,511,000 for the three and six months ended June 30, 2000, respectively. Operating losses from discontinued operations were $0 and $136,000 for the three and six months ended June 30, 2001, respectively, and $16,000 and $79,000 for the three and six months ended June 30, 2000, respectively. In summary, income (loss) from discontinued operations was $0 and $3,964,000 for the three and six months ended June 30, 2001, respectively, and $(16,000) and $(79,000) for the three and six months ended June 30, 2000, respectively.
In August 1999, the Company sold 500,000 warrants to purchase the Company’s stock to Paradigm Group, LLC, a private investment company. In February 2000, the Company received notice that Paradigm Group, LLC exercised all of their warrants to purchase the Company’s common stock. The holders of the warrants were required to pay the exercise price when the registration of the underlying shares became effective which was in December 2000. In August 2000, the Company received a summons and complaint from Paradigm Group, LLC naming the Company as a defendant. The suit, filed in the Circuit Court of Cook County, Illinois, alleged breach of contract claims and fraud against the Company in connection with the sale by the Company to the Paradigm Group, LLC of the above warrants, the exercise of those warrants by Paradigm Group, LLC and a delay in the registration of those shares with the U. S. Securities and Exchange Commission. In December 2000, Paradigm Group, LLC withdrew this lawsuit. In the fourth quarter of 2000, the Company expensed approximately $265,000 of costs related to these warrants and the registration of the underlying shares. On June 15, 2001 the Company and Paradigm Group, LLC entered into an agreement to permanently settle their disputes. Under the terms of the agreement, Paradigm Group, LLC rescinded their exercise of the common stock purchase warrants, which have since expired, and the Company retains the 500,000 shares associated with their exercise. These shares were included in the total shares outstanding as well as in the calculation of earnings (loss) per share from February 17, 2000 (the date of exercise) through June 15, 2001 (the date of the agreement). As of June 30, 2001 these shares are legally considered treasury stock however the Company intends to take the necessary measures to cancel these shares and restore these shares to authorized only status.
Overview
On February 20, 2001, the Company sold certain assets and liabilities of its wholly-owned subsidiary, BBI Clinical Laboratories (“BBICL”), to a third party for an original purchase price of $9,500,000, of which $900,000 was placed in escrow subject to certain post closing adjustments. The escrow amount has been adjusted by $206,000 to reflect a post closing adjustment in favor of the purchaser and for $12,000 interest earned. Additional information relative to this transaction is contained hereunder in the caption entitled “Discontinued Operations.” Prior period data has been reclassified to conform to the current format of presentation.
In November 2000, Panacos Pharmaceuticals, Inc (“Panacos”), formerly a subsidiary of the Company, sold a majority of its equity to third party investors. This transaction reduced the Company’s ownership in Panacos to 30.5% all of which is held in non-voting preferred stock. As a result, the Company no longer consolidates the results of Panacos in its financial reporting.
Revenue
Total revenue from continuing operations decreased 0.6%, or $35,000, to $5,500,000 in 2001 from $5,535,000 in 2000. The decrease in revenue was the result of a decrease in product revenue of 7.3%, or $260,000, to $3,280,000 in 2001 from $3,540,000 in 2000. This decrease in product revenue was partially offset by an increase in service revenue of 11.3%, or $225,000, to $2,220,000 in 2001 from $1,995,000 in 2000.
Product Revenue. The product revenue decrease was primarily attributable to a $453,000 decrease at the Diagnostics segment. Diagnostics product revenue, which was slightly higher than the first quarter of 2001, compared unfavorably with the second quarter of 2000 due to a large nonrecurring OEM panel order that shipped in the second quarter of 2000. This was partially offset by a 26% increase or $131,000 in product sales by the Laboratory Instrumentation segment due to strong sales to several existing customers.
Service Revenue. The Biotech segment experienced a 9% increase in repository services, coupled with an increase in revenues recorded in the second quarter of 2001 related to work initiated on a vaccine contract by a new collaborator. Service revenue recognized in the second quarter of 2000 included $69,000 of funding received by Panacos for drug discovery activities; as noted above, the Company no longer consolidates the results of operations of Panacos.
Gross Profit
Overall gross profit increased 3.6%, or $78,000, to $2,238,000 in the second quarter of 2001 from $2,160,000 for the same period last year. Product gross profit decreased 0.3%, or $54,000, to $1,762,000 in 2001 from $1,816,000 for 2000 on lower product sales; however, product gross margin increased to 53.7% in 2001 from 51.3% in 2000. Services gross profit increased $133,000 to $477,000 in 2001 from $344,000 for 2000 and service gross margin increased to 21.5% in 2001 from 17.2% in 2000.
Product Gross Margin
The increase in product gross margin was achieved at both manufacturing segments. At the Diagnostics segment, a greater proportion of second quarter 2001 revenue was derived from higher margin catalog products. This was coupled with a gross margin increase at the Laboratory Instrumentation segment driven by both higher revenue and the cost reduction plan implemented last September.
Service Gross Margin
The increase in service gross margin from 17.2% to 21.5% was due primarily to increased contract revenue at the Biotech segment.
Research and Development
Research and development expenditures decreased 13.3%, or $87,000, to $569,000 in 2001 from $656,000 in 2000. The second quarter of 2000 included $185,000 of research and development expenses associated with Panacos, the results of which are no longer included in the Company’s results of operation as discussed above. This was partially offset by higher research and development spending at both the Diagnostics and PCT segments.
Selling and Marketing
Selling and marketing expenses decreased by 3.1%, or $22,000, to $690,000 in 2001 from $712,000 in 2000. A decrease in sales commissions earned was partially offset by an increase in headcount associated with filling several sales and marketing positions in the latter part of 2000 at the Diagnostic segment.
General and Administrative
General and administrative costs declined 14.0%, or $145,000, to $891,000 in 2001 from $1,036,000 in 2000. This decrease was primarily a result of four factors. First, there have been headcount reductions at Corporate, and the Diagnostics and Laboratory Instrumentation segments. Second, a portion of the decline was associated with the September 2000 write down of goodwill at the Laboratory Instrumentation segment, saving $31,000 of amortization expense compared to the second quarter of 2000. Third, in the second quarter the company increased its provision for doubtful accounts by $82,000 based on a recent significant deterioration in the financial condition of a customer of the Diagnostics segment. Finally, in the second quarter of 2001, the Company reversed $80,000 of expenses previously accrued in year 2000, based on the June 2001 legal settlement reached with Paradigm Group, LLC, as discussed further in the accompanying footnotes to the financial statements.
Operating Loss
The Company recorded operating income from continuing operations of $89,000 in the second quarter of 2001 versus an operating loss from continuing operations of $(244,000) in 2000, with all operating business segments (Diagnostics, Biotech and Laboratory Instrumentation) achieving profitability prior to corporate allocations. The Diagnostics segment’s operating income was relatively unchanged as a decline in product revenue and an increased absorption of corporate overhead by this segment (as explained further hereunder) was offset by an increase in product gross margin associated with the mix of higher margin product items and significantly lower general and administrative costs. The Biotech segment’s operating loss was $(39,000) in 2001 as compared to an operating profit of $136,000 in 2000, due to lower product gross margins and an increased absorption of corporate overhead as explained further hereunder. The Laboratory Instrumentation segment’s operating loss decreased to $(34,000) for 2001 as compared to a loss for 2000 of $(304,000), due to a higher level of product sales and gross margin, and the impact in the second quarter of 2001 operating results of the September 2000 cost reduction plan. In the second quarter of 2000, Panacos incurred a $231,000 operating loss; the Company no longer consolidates the results of operation of Panacos subsequent to November 2000 as previously discussed. In the first quarter of 2001, the Company adjusted its absorption of corporate overhead based upon a revised corporate structure effective in year 2001. The present corporate structure reflects the Company’s implementation, in the latter part of year 2000, of a cost reduction plan at the Laboratory Instrumentation segment, the Company’s spin-off of Panacos as an independent company, and the Company’s decision to exit the Clinical Laboratory testing business. The latter item is reflected as discontinued operations in the accompanying financial statements. In accordance with generally accepted accounting principles, the Company ceased allocating corporate overhead to the Clinical Laboratory testing business for all periods presented. This adjustment results in the Diagnostics unit absorbing a large portion of corporate overhead which in prior years would have been allocated to Panacos and the Clinical Laboratory testing business.
Interest Expense
Interest expense decreased from $211,000 in the second quarter of 2000 to $63,000 in the second quarter of 2001. The decrease is associated with interest expense incurred on the Company’s line of credit which was in effect for the second quarter of 2000 but was repaid and terminated by the Company in February 2001.
Income Taxes
In the third quarter of 2000, the Company established a full valuation allowance for its deferred tax assets in accordance with Statement of Financial Accounting Standards No. 109 and in consideration of three consecutive years of losses; accordingly, the Company has not subsequently recognized an income tax benefit associated with losses from continuing operations as these tax assets have been fully reserved for. The Company recorded approximately $5,900 of state income tax expense in the second quarter of 2001; in the second quarter of 2000, the Company recorded an income tax benefit at a rate of 38%.
Loss from Continuing Operations
Income from continuing operations amounted to $38,000 for the quarter ended June 30, 2001 as compared to a loss of $(276,000) for the same period last year, as a result of the items discussed above.
Discontinued Operations
Operating income (loss) from discontinued operations amounted to $0 and $(16,000) for the three months ended June 30, 2001 and 2000, respectively. In accordance with a transition services agreement, the Company is required to operate the Clinical Laboratory testing business in a normal fashion for a minimum of six months (with 30 day renewal options) subsequent to the sale, but in no event beyond December 31, 2001; all of the revenues generated by, and substantially all costs associated with operating the business subsequent to February 20, 2001 are the responsibility of the purchaser.
Summary
The Company had net income of $38,000 in the second quarter of 2001 as compared to a net (loss) of $(292,000) in the second quarter of 2000. The second quarter of 2001 includes a significantly lower level of operating losses at the Laboratory Instrumentation segment, and no operating loss for Panacos, whereas the second quarter of 2000 included an operating loss of $231,000 associated with Panacos; the Company no longer consolidates the results of operation of Panacos subsequent to November 2000 as previously discussed. The net income per share computation for the second quarter of 2001 reflects the issuance of 60,029 additional shares of common stock associated with the exercise of stock options and warrants. In addition, on June 15, 2001 the Company and Paradigm Group, LLC entered into an agreement to permanently settle their disputes. Under the terms of the agreement, Paradigm Group, LLC rescinded their exercise of the common stock purchase warrants, which have since expired, and the Company retains the 500,000 shares associated with their exercise. These shares were included in the total shares outstanding as well as in the calculation of earnings (loss) per share from February 17, 2000 (the date of exercise) through June 15, 2001 (the date of the agreement). As of June 30, 2001 these shares are legally considered treasury stock however the Company intends to take the necessary measures to cancel these shares and restore these shares to authorized only status.
Revenue
Total revenue from continuing operations increased 4.1%, or $408,000, to $10,482,000 in 2001 from $10,074,000 in 2000. The increase in revenue was the result of an increase in product revenue of 5.0%, or $305,000, to $6,442,000 in 2001 from $6,137,000 in 2000. This increase in revenue was coupled with an increase in service revenue of 2.6%, or $103,000, to $4,040,000 in 2001 from $3,937,000 in 2000.
Product Revenue. The product revenue increase was primarily attributable to a $277,000 increase in sales of Basematrix products in the Diagnostics segment coupled with a $77,000 increase in product sales at the Laboratory Instrumentation segment due to strong sales to several existing customers.
Service Revenue. The Biotech segment experienced a 24.6% increase in repository service revenues coupled with an increase in revenues related to work initiated on a vaccine contract by a new collaborator. Service revenue recognized in the first half of 2000 also included $138,000 of funding received by Panacos for drug discovery activities; as noted above, the Company no longer consolidates the results of operations of Panacos.
Gross Profit
Overall gross profit increased 15.0%, or $569,000, to $4,360,000 in the first half of 2001 from $3,791,000 for the same period last year. Product gross profit increased 7.2%, or $224,000, to $3,343,000 in 2001 from $3,119,000 for 2000; product gross margin increased slightly to 51.9% in 2001 from 50.8% in 2000. Services gross profit increased $345,000 to $1,017,000 in 2001 from $672,000 for 2000 and service gross margin increased to 25.2% in 2001 from 17.1% in 2000.
Product Gross Margin
A slight increase in product gross margin at the Diagnostics segment, was derived from increased sales of higher margin catalog products. This was coupled with a gross margin increase at the Laboratory Instrumentation segment driven by the cost reduction plan implemented last September.
Service Gross Margin
The increase in service gross margin was due to increased contract revenues at the Biotech segment.
Research and Development
Research and development expenditures decreased 14.1%, or $190,000, to $1,159,000 in 2001 from $1,349,000 in 2000. The first half of 2000 included $374,000 of research and development expenses associated with Panacos, the results of which are no longer included in the Company’s results of operation as discussed above. This was partially offset by higher research and development spending at the Diagnostics segment.
Selling and Marketing
Selling and marketing expenses increased by 6.7%, or $90,000, to $1,441,000 in 2001 from $1,351,000 in 2000. This increase was a result of filling several sales and marketing positions in the latter part of 2000 at the Diagnostic segment.
General and Administrative
General and administrative costs declined 11.2%, or $241,000, to $1,920,000 in 2001 from $2,161,000 in 2000. This decrease was primarily a result of four factors. First, there have been headcount reductions at Corporate, and the Diagnostics and Laboratory Instrumentation segments. Second, a portion of the decline was associated with the September 2000 write down of goodwill at the Laboratory Instrumentation segment, saving $62,000 of amortization expense compared to the first half of 2000. Third, in the second quarter the company increased its provision for doubtful accounts by $82,000 based on a recent significant deterioration in the financial condition of a customer of the Diagnostics segment. Finally, in the second quarter of 2001, the Company reversed $80,000 of expenses previously accrued in year 2000, based on the June 2001 legal settlement reached with Paradigm Group, LLC, as discussed further in the accompanying footnotes to the financial statements.
Operating Loss
Operating loss from continuing operations decreased to $(160,000) in 2001 versus $(1,070,000) in 2000. The Diagnostics segment’s operating income decreased to $669,000 in 2001 from $717,000 in 2000, due to a decline in total revenue coupled with an increased absorption of corporate overhead by this segment as explained further hereunder. This was partially offset by significantly lower segment general and administrative costs. The Laboratory Instrumentation segment had an operating loss of $157,000 for 2001 versus a loss for 2000 of $590,000; the first half of 2001 operating results reflect the impact of the September 2000 cost reduction plan. The operating loss of the PCT segment decreased to $582,000 in 2001 from $650,000 in 2000, as increased research funding revenues offset an increased absorption of corporate overhead by this segment as explained further hereunder. Effective January 2001, the Company adjusted its absorption of corporate overhead based upon a revised corporate structure effective in year 2001. The present corporate structure reflects the Company’s implementation, in the latter part of year 2000, of a cost reduction plan at the Laboratory Instrumentation segment, the Company’s spin-off of Panacos as an independent company, and the Company’s decision to exit the Clinical Laboratory testing business. The latter item is reflected as discontinued operations in the accompanying financial statements. In accordance with generally accepted accounting principles, the Company ceased allocating corporate overhead to the Clinical Laboratory testing business for all periods presented. This adjustment results in the Diagnostics unit absorbing a large portion of corporate overhead which in prior years would have been allocated to Panacos and the Clinical Laboratory testing business.
Interest Expense
Interest expense decreased from $404,000 in 2000 to $309,000 in 2001. The major portion of the decrease is associated with reduced interest expense incurred in the first half of 2001 on the Company’s line of credit, which was in effect for the entire first half of 2000 but was repaid and terminated by the Company in February 2001 as discussed further in the section “Discontinued Operations” hereunder. In the first quarter of 2001, the Company also redeemed the remaining $2,040,000 (face value) of outstanding 3% Senior Subordinated Convertible Debentures (the “Debentures”) plus accrued interest and a premium of $190,000 (which was charged to interest expense). The pro-rata portion of unamortized original issue discount, debt issuance and warrant related costs associated with the redeemed Debentures, amounting to approximately $377,000, is included in the loss on extinguishment of the Debentures. Substantially offsetting this loss is the Company’s reversal of approximately $528,000 of interest expense previously recorded in 2000 associated with the amortization of the Debentures beneficial conversion feature. Additional interest expense was incurred in the first six months of 2001 associated with the Company obtaining a $2,447,000 mortgage on its West Bridgewater MA facility in April 2000, together with interest on $3,250,000 of 3% Senior Subordinated Convertible Debentures which were issued in August 2000.
Income Taxes
In the third quarter of 2000, the Company established a full valuation allowance for its deferred tax assets in accordance with Statement of Financial Accounting Standards No. 109 and in consideration of three consecutive years of losses; accordingly, the Company has not subsequently recognized an income tax benefit associated with the loss from continuing operations in the first half of 2001 as these tax assets have been fully reserved for. The Company has recorded approximately $16,000 of state income tax expense in the first half of 2001; in the first half of 2000, the Company recorded an income tax benefit at a rate of 38%.
Loss from Continuing Operations
Loss from continuing operations decreased to $(449,000) for the six months ended June 30, 2001 from $(884,000) for the same period last year, as a result of the items discussed above.
Discontinued Operations
On February 20, 2001, the Company sold
certain assets and liabilities of its wholly-owned subsidiary BBICL to a third
party for an original purchase price of $9,500,000, of which $900,000 was placed
in escrow subject to certain post closing adjustments. The escrow amount has
been adjusted by $206,000 to reflect a post closing adjustment in favor of the
purchaser and for $12,000 interest earned. The Company has retained certain
other assets and liabilities of BBICL, primarily property plant and equipment
together with a facility lease subsequent to the closing date, which the Company
intends to liquidate throughout the remainder of year 2001 as part of its
decision to exit this segment of the business. The Company has written all of
the retained assets down to their estimated net realizable value.
The Company has
recorded a gain of $4,100,000 net of taxes of $1,364,000 in the first
quarter of 2001, subject to post closing adjustments. The Company expects to
utilize approximately $5,200,000 of prior period net operating loss
carryforwards, previously reserved for by the Company in year 2000, to partially
offset the tax effect of this gain. Additionally, the Company has taken a tax
benefit of $364,000 related to stock option exercises that was not previously
recorded as the Company was in a loss position. This tax benefit was recorded as
a credit to additional paid-in capital in the first half of 2001.
The Company has
recorded its estimate of remaining short and long term accrued liabilities
to exit the Clinical Laboratory testing business, totaling approximately
$2,780,000 as of June 30, 2001. The major components of this accrual are
estimated income taxes ($817,000), severance and other employee related costs
($669,000), estimated lease exit costs ($773,000) and potential health care
claim adjustments ($218,000); with the remainder for professional fees,
potential additional post-closing adjustments, and other miscellaneous costs
associated with exiting this business segment.
Revenues from
discontinued operations net of intercompany eliminations were $973,000 for
the period from January 1, 2001 to February 20, 2001 and $2,219,000 and
$4,511,000 for the three and six months ended June 30, 2000, respectively.
Operating (losses) from discontinued operations were $0 and $(136,000) for the
three and six months ended June 30, 2001, respectively, and $(16,000) and
$(79,000) for the three and six months ended June 30, 2000, respectively. In
summary, income (loss) from discontinued operations was $0 and $3,964,000 for
the three and six months ended June 30, 2001, respectively, and $(16,000) and
$(79,000) for the three and six months ended June 30, 2000, respectively.
In accordance with a
transition services agreement, the Company is required to operate the
business in a normal fashion for a minimum of six months subsequent to the sale
(with 30 day renewal options) but in no event beyond December 31, 2001; all of
the revenues generated by, and substantially all costs associated with operating
the business subsequent to the closing date of the transaction are the
responsibility of the purchaser. A portion of the proceeds from this sale were
used to redeem all outstanding Debentures and to retire the Company’s line
of credit.
Summary
The Company had net income of $3,516,000 in
2001 as compared to a net loss of $(963,000) in 2000. In the first half of 2001,
the Company recorded an after-tax gain of $3,964,000 associated with
discontinued operations, coupled with a significantly lower operating loss at
the Laboratory Instrumentation segment. In the first half of last year, the
Company incurred a higher level of operating losses which included $532,000
associated with Panacos. The Company no longer consolidates the results of
operation of Panacos subsequent to November 2000 as discussed further above. The
operating losses in year 2000 were partially offset by a benefit from income
taxes, which the Company fully reserved for and ceased recording in the third
quarter of 2000.
The income (loss) per
share computation for the first half of 2001 reflects both the issuance of
801,325 additional shares of common stock in the first quarter of 2001, as
certain holders of the Debentures exercised their rights to convert $1,210,000
of such Debentures into shares of the Company’s common stock, and the
issuance of 68,355 additional shares of common stock associated with the
exercise of stock options, warrants and purchases made pursuant to the employee
stock purchase plan. In addition, on June 15, 2001 the Company and Paradigm
Group, LLC entered into an agreement to permanently settle their disputes. Under
the terms of the agreement, Paradigm Group, LLC rescinded their exercise of the
common stock purchase warrants, which have since expired, and the Company
retains the 500,000 shares associated with their exercise. These shares were
included in the total shares outstanding as well as in the calculation of
earnings (loss) per share from February 17, 2000 (the date of exercise) through
June 15, 2001 (the date of the agreement). As of June 30, 2001 these shares are
legally considered treasury stock however the Company intends to take the
necessary measures to cancel these shares and restore these shares to authorized
only status.
The Company's working capital position increased to $8,321,000 as of June 30, 2001 from $3,596,000 as of December 31, 2000. From March 31, 2000 through February 2001 the Company’s working capital position was adversely affected by the classification of its line-of-credit as short-term debt. The Company reclassified the debt to short term because in the first quarter of 2000, it violated a financial covenant limiting the amount of allowable losses. In February 2001, utilizing proceeds generated by the sale of certain assets of BBICL as discussed above, the Company paid off in full the $5,763,000 outstanding balance (plus accrued interest), thereby terminating this line of credit. There were no payment defaults at any time on this line of credit.
In August 2000, the Company issued $3,250,000 of 3% Senior Subordinated Convertible Debentures (the “Debentures”) due August 25, 2003. Net proceeds to the Company amounted to approximately $2,871,000 after deduction of original issue discount of $162,500 and associated closing costs of $216,500. For accounting purposes, a portion of the cash proceeds, amounting to $327,000, was allocated to the relative fair value of warrants issued in conjunction with these Debentures. In the first quarter of 2001, certain holders of the Debentures exercised their rights to convert $1,210,000 of such Debentures into shares of the Company’s common stock, in accordance with the conversion formula.
In the first quarter of 2001, certain holders of these Debentures exercised their rights to convert $1,210,000 of such Debentures into shares of the Company’s common stock. These conversions resulted in the issuance of 801,325 shares of common stock. The pro-rata portion of the unamortized original issue discount, debt issuance and warrant related costs amounting to approximately $231,000 was charged against additional paid-in capital. In addition, the Company redeemed the remaining $2,040,000 (face value) of Debentures plus accrued interest and a premium of $190,000 (which was charged to interest expense). The pro-rata portion of unamortized original issue discount, debt issuance and warrant related costs associated with the redeemed Debentures amounting to approximately $377,000 has been included in the loss on extinguishment of the Debentures. Partially offsetting this loss is the Company’s reversal of approximately $528,000 of interest expense previously recorded in 2000 associated with the amortization of the Debentures beneficial conversion feature. Accordingly, the Company has recorded a loss of approximately $(39,000) relative to the early extinguishment of the Debentures. As a result of the conversions and redemptions noted above, none of the Debentures remain outstanding subsequent to February 27, 2001.
Net cash used in operations for the six months ended June 30, 2001 was $121,000 as compared to $1,336,000 during the same period last year. The decrease in cash used in operations during the first half of 2001is primarily associated with increased cash collections on accounts receivable and the collection of an income tax refund.
Net cash used in investing activities was $132,000 in the first half of 2001 versus $881,000 in the comparable prior year period. The decrease of cash used for investing was due to the completion of the second phase of improvements at the Company’s Frederick, MD and West Bridgewater, MA facilities and a decision by management to control capital expenditures.
Cash used in financing activities was $7,303,000 in the first half of 2001 versus cash provided of $1,817,000 for the prior year period. In the first half of 2001, the Company used proceeds from the sale of the business and certain assets of BBICL to pay off in full the remaining $5,762,635 balance on its line of credit and retire all remaining Debentures. Cash provided by financing in the first half of 2000 consisted of approximately $656,000 of cash received from the exercise of stock options and warrants and net proceeds from a $2,477,000 mortgage on its West Bridgewater, MA facility in April 2000. This was partially offset by $1,326,000 of repayments on the Company’s line of credit.
In the period from January 1, 2001 through August 3, 2001 the Company has received proceeds totaling $302,000 ($165,000 through June 30, 2001), associated with the exercise of stock options and warrants, which have resulted in 116,350 new shares of common stock being issued. As of June 30, 2001, the Company had existing cash balances approximating $1,866,000 (excluding $706,000 of cash held in escrow relating to the sale of certain BBICL assets as discussed herein) and believes this amount, coupled with internally generated funds from operations, will be sufficient to fund operations and anticipated capital expenditures for the remainder of the year. The Company continually evaluates financing options, as well as other strategic alternatives, in order to maximize shareholder value.
This Quarterly Report on Form 10-Q contains forward-looking statements concerning the Company's financial performance and business operations. The Company wishes to caution readers of this Quarterly Report on Form 10-Q that actual results might differ materially from those projected in any forward-looking statements.
Factors which might cause actual results to differ materially from those projected in the forward-looking statements contained herein include the following: due to difficulties in the implementation of its strategies, the Company may not be able to return to operating profitability for the full year 2001; the financial results of the quarter and six months ended June 30, 2001 are not necessarily indicative of future results because future revenues may not meet expectations due to changes in customer needs and technological innovations and expenses may be higher than anticipated because costs may increase; the Company may not be successful in developing Pressure Cycling Technology (PCT) into commercially successful products, or such development may take longer than expected; the Company’s inability to develop the end-user market for quality control products; the Company’s inability to integrate the business of Source Scientific, Inc. into the Company's business and to grow the sales of Source Scientific, Inc. to the extent anticipated by the September 2000 downsizing of this segment of the business; the uncertainty of the renewal and full funding of contracts with National Institutes of Health (NIH), National Heart, Lung and Blood Institute (NHLBI) and other government agencies; the Company’s inability to obtain an adequate supply of the unique and rare specimens of plasma and serum necessary for certain of its products; and the potential for significant reductions in purchases by any of the Company's major customers; if expenses are higher than anticipated, or if revenues are lower than anticipated, the Company will require additional capital sooner than expected and there can be no assurance that the Company will be able to obtain additional capital on acceptable terms. Certain of these and other factors which might cause actual results to differ materially from those projected are more fully set forth under the caption "Risk Factors" in the Company's most recent Registration Statements on Form S-3 (SEC File No.’s 333-94379 and 333-46426) and in its annual report on Form 10-K for the year ended December 31, 2000 and its quarterly report on form 10-Q for the quarter ended March 31, 2001.
There have been no material changes in the reported market risks since December 31, 2000.
In August 1999, the Company sold 500,000 warrants to purchase the Company’s stock to Paradigm Group, LLC, a private investment company. In February 2000, the Company received notice that Paradigm Group, LLC exercised all of their warrants to purchase the Company’s common stock. The holders of the warrants were required to pay the exercise price when the registration of the underlying shares became effective which was in December 2000. In August 2000, the Company received a summons and complaint from Paradigm Group, LLC naming the Company as a defendant. The suit, filed in the Circuit Court of Cook County, Illinois, alleged breach of contract claims and fraud against the Company in connection with the sale by the Company to the Paradigm Group, LLC of the above warrants, the exercise of those warrants by Paradigm Group, LLC and a delay in the registration of those shares with the U. S. Securities and Exchange Commission. In December 2000, Paradigm Group, LLC withdrew this lawsuit. In the fourth quarter of 2000, the Company expensed approximately $265,000 of costs related to these warrants and the registration of the underlying shares. On June 15, 2001 the Company and Paradigm Group, LLC entered into an agreement to permanently settle their disputes. Under the terms of the agreement, Paradigm Group, LLC rescinded their exercise of the common stock purchase warrants, which have since expired, and the Company retains the 500,000 shares associated with their exercise. These shares were included in the total shares outstanding as well as in the calculation of earnings (loss) per share from February 17, 2000 (the date of exercise) through June 15, 2001 (the date of the agreement). As of June 30, 2001 these shares are legally considered treasury stock however the Company intends to take the necessary measures to cancel these shares and restore these shares to authorized only status.
In accordance with the terms of the Company’s mortgage with a bank, payment of dividends on common stock is not permitted. See also Item 3 hereunder.
Not Applicable
The Company held its Annual Meeting of Stockholders on June 21, 2001 (the "Meeting"). A total of 5,120,072 shares, or 85.8%, of the Common Stock issued, outstanding and entitled to vote as of the record date, were represented at the meeting by proxy. At the meeting, one proposal was acted upon. The result of the proposal was as follows:
1. William A. Wilson was elected a Class II Director of the Company, to serve as such until the Year 2004 Annual Meeting of Stockholders and until a successor has been duly elected and qualified, with 4,780,817 shares voting in favor, 339,255 votes withheld.
The terms of office of Directors Richard T. Schumacher, Kevin W. Quinlan, Francis E. Capitanio and Calvin A Saravis, continued after the Meeting.
Not Applicable
| 2 | Asset Purchase Agreement dated February 20, 2001, by and between BBI Clinical Laboratories, Inc., Boston Biomedica, Inc. and Specialty Laboratories, Inc. (Annexes, Exhibits and Schedules are omitted pursuant to Item 601(b)(2) of Regulation S-K. Boston Biomedica, Inc. agrees, however, to furnish supplementary a copy of such omitted items to the Commission upon request.) * |
| 3.1 | Amended and Restated Articles of Organization of the Company** |
| 3.2 | Amended and Restated Bylaws of the Company** |
| 4.1 | Specimen Certificate for Shares of the Company's Common Stock** |
| 4.2 | Description of Capital Stock (contained in the Restated Articles of Organization of the Company filed as Exhibit 3.1)** |
| * | In accordance with Rule 12b-32 under the Securities Exchange Act of 1934, as amended, reference is made to the documents previously filed with the Securities and Exchange Commission, as exhibits to the Company's Report on Form 8-K filed March 8, 2001, which documents are hereby incorporated by reference. The number set forth herein is the number of the Exhibit in said Form 8-K. |
| ** | In accordance with Rule 12b-32 under the Securities Exchange Act of 1934, as amended, reference is made to the documents previously filed with the Securities and Exchange Commission, as exhibits to the Company's Registration Statement on Form S-1 (Registration No. 333-10759), which documents are hereby incorporated by reference. The number set forth herein is the number of the Exhibit in said registration statement. |
None.
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
BOSTON BIOMEDICA, INC.
(Registrant)
Date:
August 13, 2001
By: /s/ Kevin W. Quinlan
Kevin W. Quinlan,
President and Chief Operating Officer, and Treasurer
(Principal Accounting and Financial Officer)